Mammaglobin mRNA detection method and reagent thereof
A detection method and a technology of a detection kit, which are applied in DNA/RNA fragments, recombinant DNA technology, microbial determination/inspection, etc., can solve the problems of high environmental purity requirements, high false positive rate of antibody specificity requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1 detection uses the preparation of MAM mRNA sample and its contrast
[0070] MDA-MB361 cells were cultured in vitro, the adherent cells were digested with trypsin to suspend the cells, the culture medium was removed by centrifugation, and then normal saline was added to prepare a single cell suspension, and a certain amount of cells (10 7 )conduct experiment. According to the manufacturer's instructions, total cellular RNA (positive for MAM mRNA expression) was extracted from MDA-MB361 cells with the total cellular RNA extraction reagent, and the optical density (OD value) at 260 and 280 nm was measured to quantify the amount of total RNA. Then the total RNA was serially diluted with TE buffer (pH7.2), and each dilution solution contained equal volumes from the equivalent of 10 5 (Group A), 10 4 (Group B), 10 3 (Group C), 10 2 (Group D) and 10 1 (Group E) total RNA amount of MDA-MB361 breast cancer cells.
[0071] According to the above method, the tot...
Embodiment 2
[0072] Synthesis and preparation of embodiment 2 aptamers
[0073] Take 12 microliters of magnetic body solution with carboxy-terminus in a 1.5ml centrifuge tube, wash with 0.1M imidazole buffer three times (each time add 150 microliters of 0.1M imidazole buffer (pH7.0) and mix well, then centrifuge. Discard the supernatant), add 300 microliters of 0.1M imidazole buffer (pH7.0) containing 0.03M EDC, shake the centrifuge tube slowly for 20 minutes, and then add 12pmol of the first aptamer (5'-aaggaagccgctgtc-3' ) was added, mixed and incubated at 37°C for 1 hour, during which the centrifuge tube was continuously shaken slowly. Place the centrifuge tube on a magnetic separator to apply magnetic force, blot the supernatant and wash it three times with washing solution (add 300 μl of washing solution (PBS solution containing 100 mM NaCl, pH 7.2) and blot dry) for three times. Unbound free first aptamer was removed, and then dissolved by adding 240 microliters of dissolving soluti...
Embodiment 3
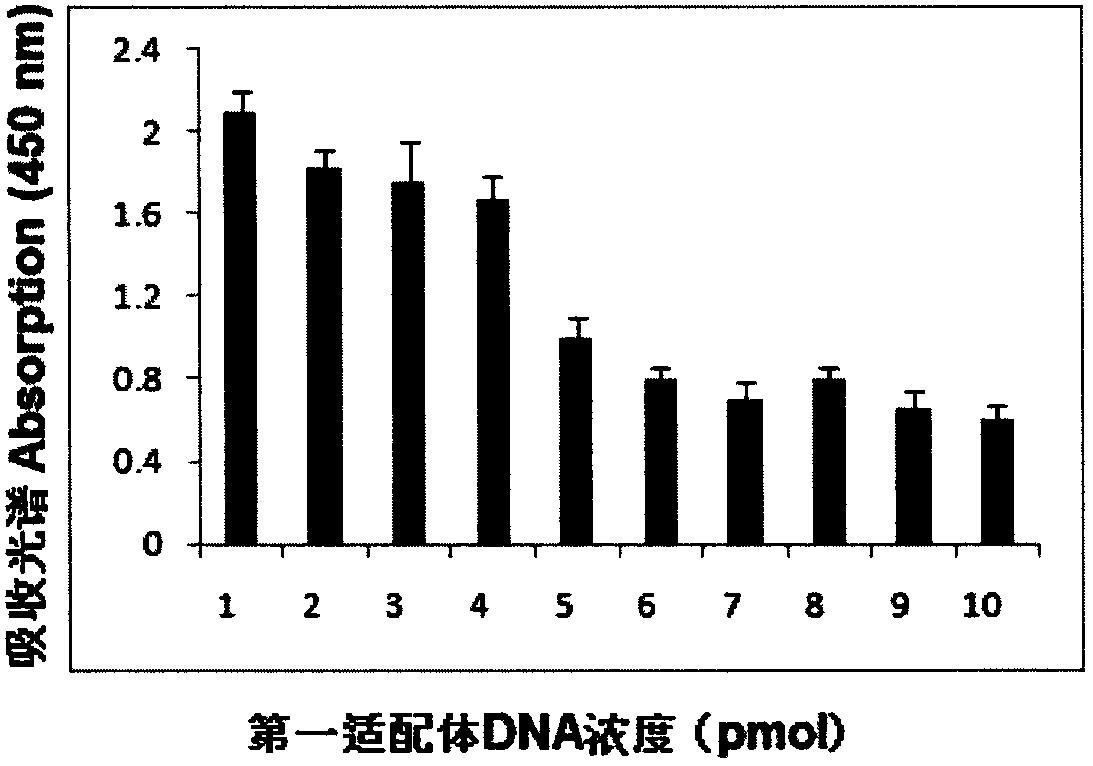
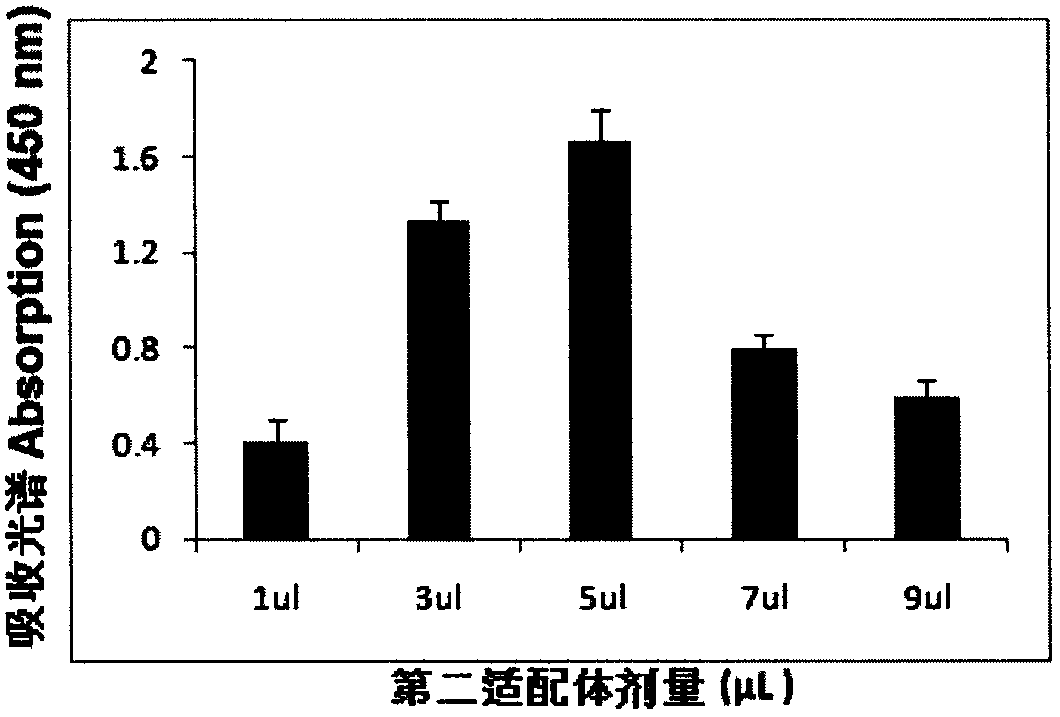
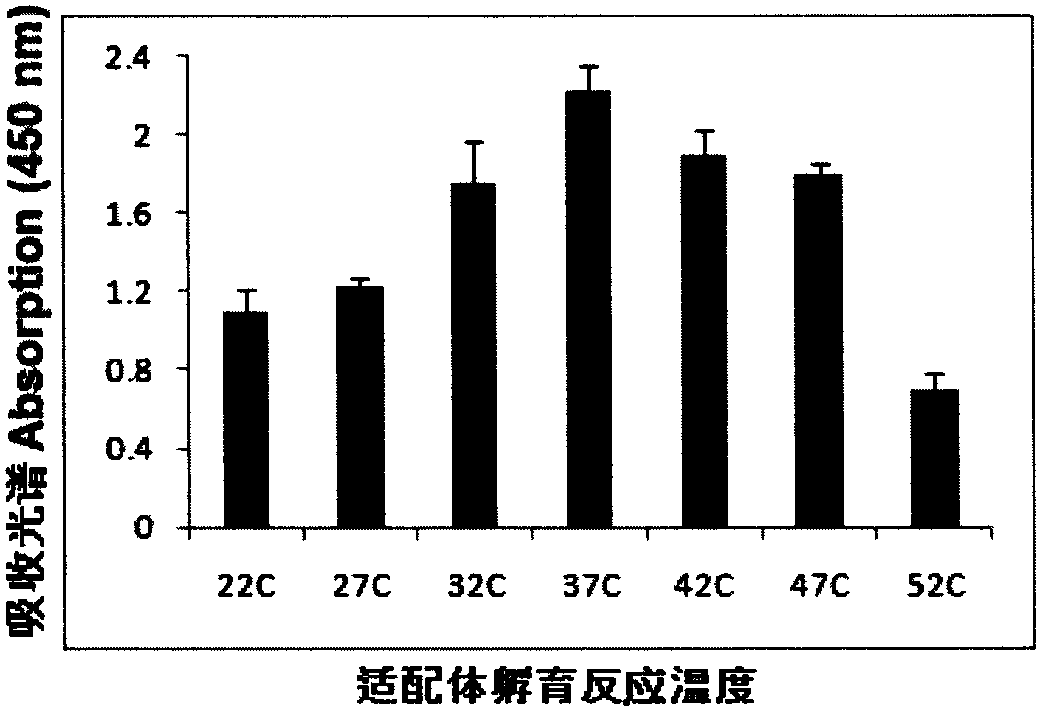
[0075] Example 3 Determination of the concentration of the first aptamer and the second aptamer
[0076] In a series of reaction tubes, the total RNA (1 microgram) of MDA-MB361 cells known to contain the target molecule (MAM mRNA) was added, and the first aptamer (2- 20 microliters, 1-10pmol) mixed, added PBS (0.1M, pH7.2) to 100 microliters, incubated at 37°C for 60 minutes; then placed the test tube on a magnetic separator to apply magnetic force, blotted the supernatant and used The washing solution was washed three times (300 microliters of washing solution (PBS solution containing 100 mM NaCl, pH 7.2) was added each time and blotted dry), 95 microliters of PBS buffer solution was added, mixed evenly, and then 5 microliters were added according to Example 2. The prepared second aptamer coupled with gold nanoparticles was incubated at 37° C. for 60 minutes, and then the test tube was placed on a magnetic separator to apply magnetic force, the supernatant was blotted, and wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com