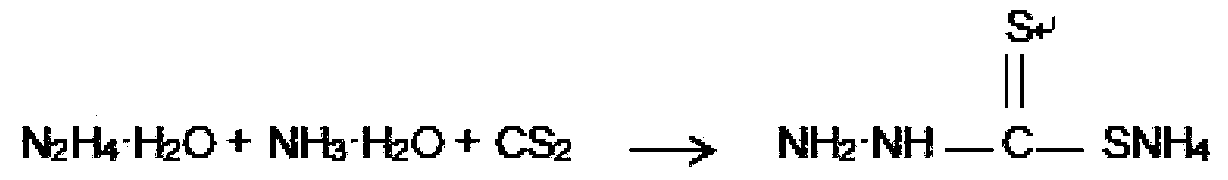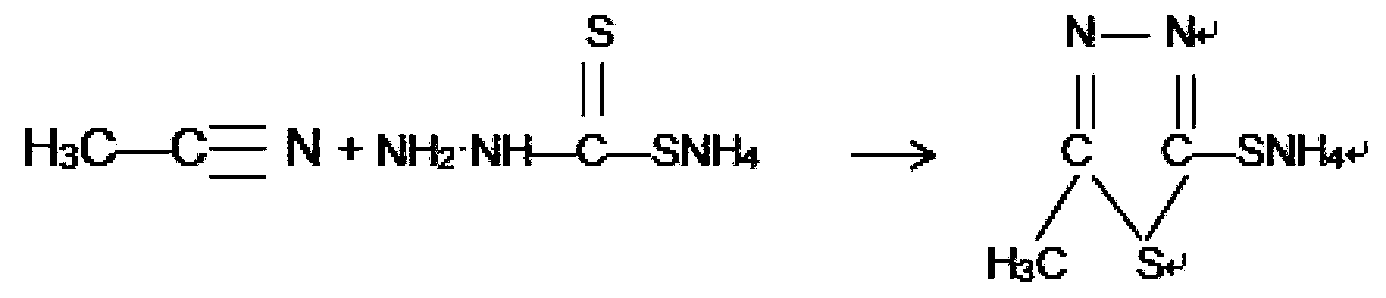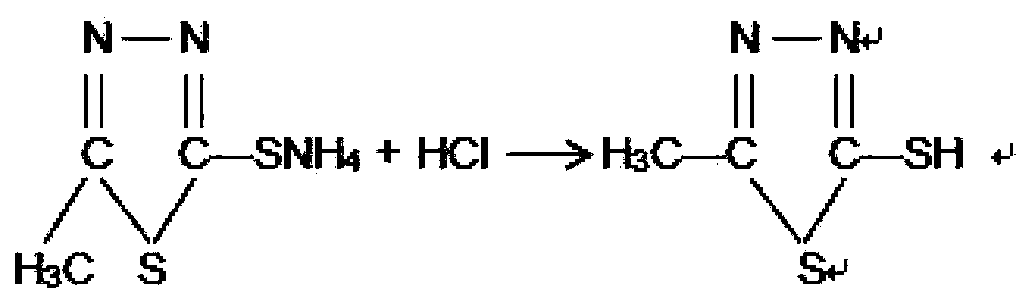Preparation method of 2-sulfydryl-5-methyl-1, 3, 4-thiadiazole
A technology of thiadiazole and methyl, which is applied in the field of preparation of 2-mercapto-5-methyl-1,3,4-thiadiazole, which can solve the problems of complex operation, high production cost and long reaction time , to achieve the effect of simplifying the process steps, reducing the cost of raw materials, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 50 grams of 100% hydrazine hydrate and 120 grams of 20% ammonia water in a 500 milliliter synthesis reaction bottle successively, and stir under ice water cooling to lower the temperature to below 5°C. Start to add 90 grams of carbon disulfide dropwise, and keep stirring at below 5° C. for 1 hour after addition. An aqueous solution of ammonium hydrazinodithioformate was obtained.
[0021] Transfer the solution obtained above into a 1000 ml reaction flask, add 100 g of acetonitrile, stir naturally for half an hour, then raise the temperature to 85-90°C and keep it warm for 1 hour. Then cool the solution down to below 40°C, add 30% hydrochloric acid dropwise in the range of 30°C to 35°C, adjust the pH to 4 to 4.5, stir for half an hour, filter with suction, and wash with water three times to obtain 130g of wet thiadiazole.
[0022] The obtained wet product was dissolved with ammonia water, decolorized, kept warm, filtered, acidified, suction filtered and dried to obt...
Embodiment 2
[0024] Put 50 grams of 100% hydrazine hydrate and 120 grams of 20% ammonia water in a 500 milliliter synthesis reaction bottle successively, and stir under ice water cooling to lower the temperature to below 5°C. Start to add 80 grams of carbon disulfide dropwise, and keep stirring at below 5° C. for 1 hour after addition. An aqueous solution of ammonium hydrazinodithioformate was obtained.
[0025] Transfer the solution obtained above into a 1000 ml reaction flask, add 100 g of acetonitrile, stir naturally for half an hour, then raise the temperature to 85-90°C and keep it warm for 1 hour. Then the solution was cooled to below 40°C, 35% hydrochloric acid was added dropwise in the range of 30°C to 35°C, the pH was adjusted to 4 to 4.5, stirred for half an hour, suction filtered, washed 3 times with water, and 142g of wet thiadiazole was obtained.
[0026] The obtained wet product was dissolved with ammonia water, decolorized, kept warm, filtered, acidified, suction filtered, ...
Embodiment 3
[0028] Put 50 grams of 100% hydrazine hydrate and 120 grams of 20% ammonia water in a 500 milliliter synthesis reaction bottle successively, and stir under ice water cooling to lower the temperature to below 5°C. Start to add 80 grams of carbon disulfide dropwise, and keep stirring at below 5° C. for 1 hour after addition. An aqueous solution of ammonium hydrazinodithioformate was obtained.
[0029] Transfer the solution obtained above into a 1000 ml reaction flask, add 105 g of acetonitrile, stir naturally for half an hour, then raise the temperature to 85-90° C. for 1 hour. Then the solution was cooled to below 40°C, 35% hydrochloric acid was added dropwise in the range of 30°C to 35°C, the pH was adjusted to 4 to 4.5, stirred for half an hour, suction filtered, and washed with water three times to obtain thiadiazole wet product 135g.
[0030] The obtained wet product was dissolved with ammonia water, decolorized, kept warm, filtered, acidified, suction filtered, and dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com