Methylprednisolone sodium succinate freeze-dried powder injection
A technology of methylprednisolone sodium succinate and freeze-dried powder injection, which is applied in the field of freeze-dried powder injection containing methylprednisolone sodium succinate, which can solve the problems of low production efficiency, low pass rate, and increased insoluble particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation process
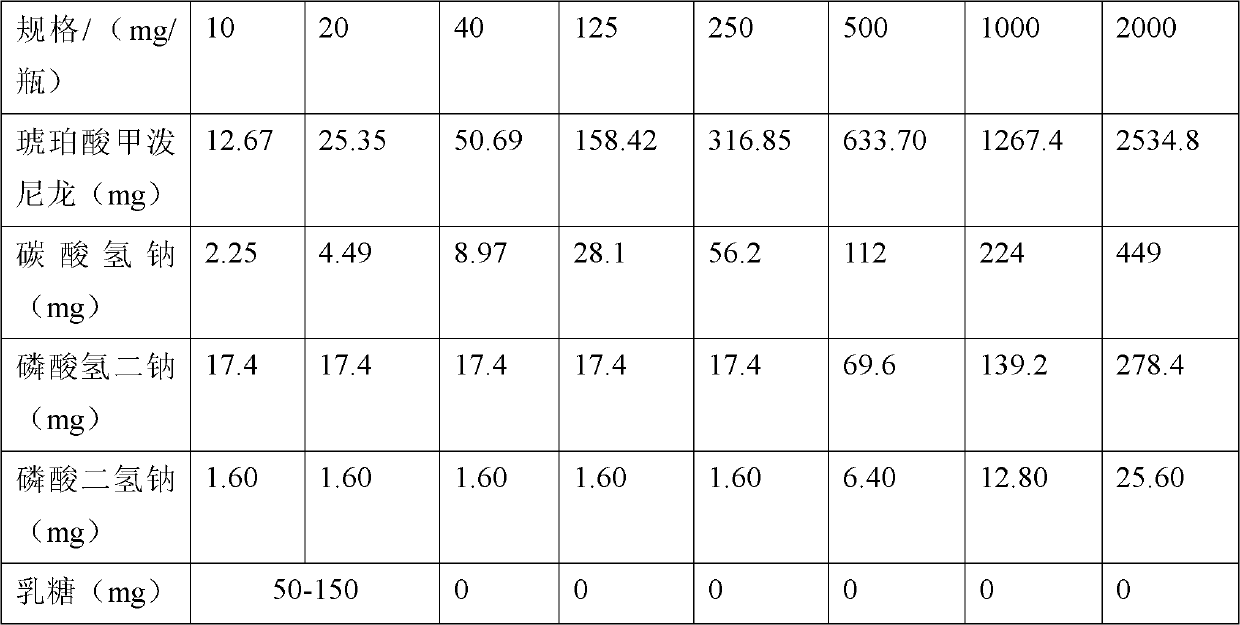
[0032] 1. Weigh the prescription amount of NaHCO 3 , add an appropriate amount of water for injection to dissolve, set aside.
[0033] 2. Weigh the prescription amount NaH 2 PO 4 ·H 2 O, add an appropriate amount of water for injection to dissolve, set aside.
[0034] 3. Weigh the prescription amount Na 2 HPO 4 , add an appropriate amount of water for injection to dissolve, set aside.
[0035] 4. Weigh the prescribed amount of lactose, add an appropriate amount of water for injection to dissolve, and set aside.
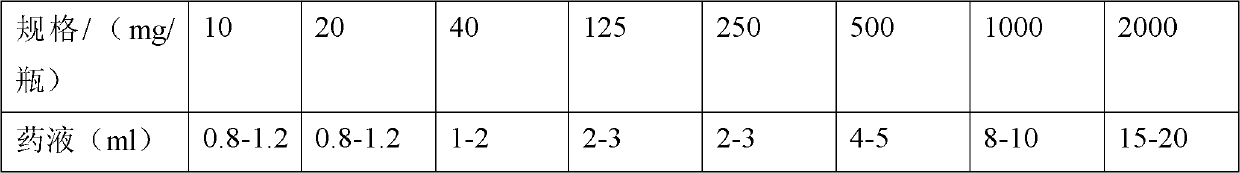
[0036] 5. Weigh the main drug of the prescribed amount, add 80% of the prescribed amount of water for injection and the prescribed amount of ethanol, stir to form a suspension, and slowly add NaHCO under constant stirring 3 solution, heated to 40-50°C in a water bath and stirred, adding NaH 2 PO 4 ·H 2 O solution, Na 2 HPO 4 Solution and lactose solution, make up the prescribed amount of water for injection, keep warm in a wat...
Embodiment 2
[0043] Preparation process is the same as embodiment 1
[0044] Freeze-drying process
[0045] 1) Pre-freeze, control the product temperature below -40°C, and keep it warm for 1 hour.
[0046] 2) Sublimation drying, turn on the vacuum pump, control the vacuum degree below 40pa, gradually raise the temperature to control the product temperature at -10°C to -8°C, keep it for 2 hours, then raise the temperature to -5°C, and keep it for 6 hours.
[0047] 4) Secondary drying, control the vacuum degree below 30pa, raise the temperature to make the product temperature rise to 33-36°C, then make the freeze-drying box reach the ultimate vacuum, and keep it warm for 2h.
[0048] 5) Release the vacuum, and cap to obtain the finished product.
Embodiment 3
[0050] 1. Weigh the prescription amount of NaHCO 3 , add an appropriate amount of water for injection to dissolve, set aside.
[0051] 2. Weigh the prescription amount NaH 2 PO 4 ·H 2 O, add an appropriate amount of water for injection to dissolve, set aside.
[0052] 3. Weigh the prescription amount Na 2 HPO 4 , add an appropriate amount of water for injection to dissolve, set aside.
[0053] 4. Weigh the main drug of the prescribed amount, add 80% of the prescribed amount of water for injection and the prescribed amount of ethanol, stir to form a suspension, and slowly add NaHCO under constant stirring 3 solution, heated to 40-50°C in a water bath and stirred, adding NaH 2 PO 4 ·H 2 O solution, Na 2 HPO 4 Solution, make up the prescription amount of water for injection, keep warm in a water bath until the solution is clear, and circulate and filter the whole amount. Filter the medicinal solution with a 0.45 μm microporous membrane, and finally fine filter with a 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com