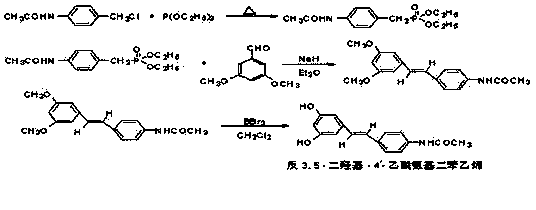
Preparation of tans-3,5- dihydroxy-4'- acetylamido-stilbene
A technology of acetamidostilbene and dihydroxyl, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, organic chemistry, etc., can solve the problems of large dosage and high cost of chemical synthesis, and achieve fast reaction speed and high selectivity. High effect with few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 500 ml three-necked flask, add p-acetamidobenzyl chloride (18.36 g, 0.1 mol) and triethyl phosphite (33.23 g, 0.2 mol) successively, continuously stir the reaction at 130°C for 4 hours, and depressurize Distill off excess triethyl phosphite, add 3.6 grams of sodium hydride solid base and 3,5-dimethoxybenzaldehyde (15.2 grams) to the residue, add 250 milliliters of diethyl ether as a solvent for the reaction, at 80 ° C The reaction was stirred at high temperature for 4 hours. After cooling, the reaction mixture was fully washed with saturated brine, several layers were separated, dried with anhydrous sodium sulfate, and diethyl ether was distilled off to obtain the solid product trans-3,5-dimethoxy- 4'-Acetamido stilbene, the solid product trans-3,5-dimethoxy-4'-acetamido stilbene was added to a three-necked flask, and 250 ml of dry dichloromethane was added as a solvent, cooled To about 0 ℃, under the protection of nitrogen, slowly add boron tribromide (28 ml,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com