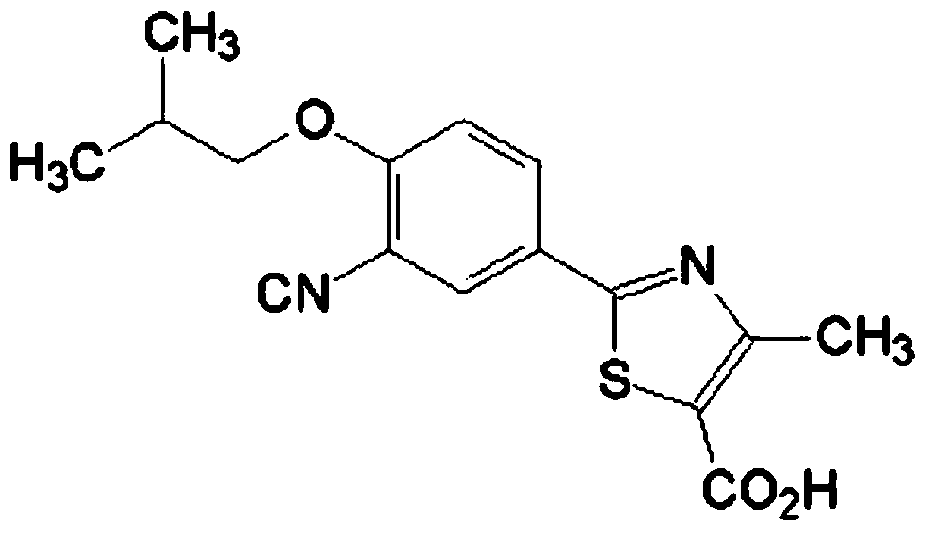
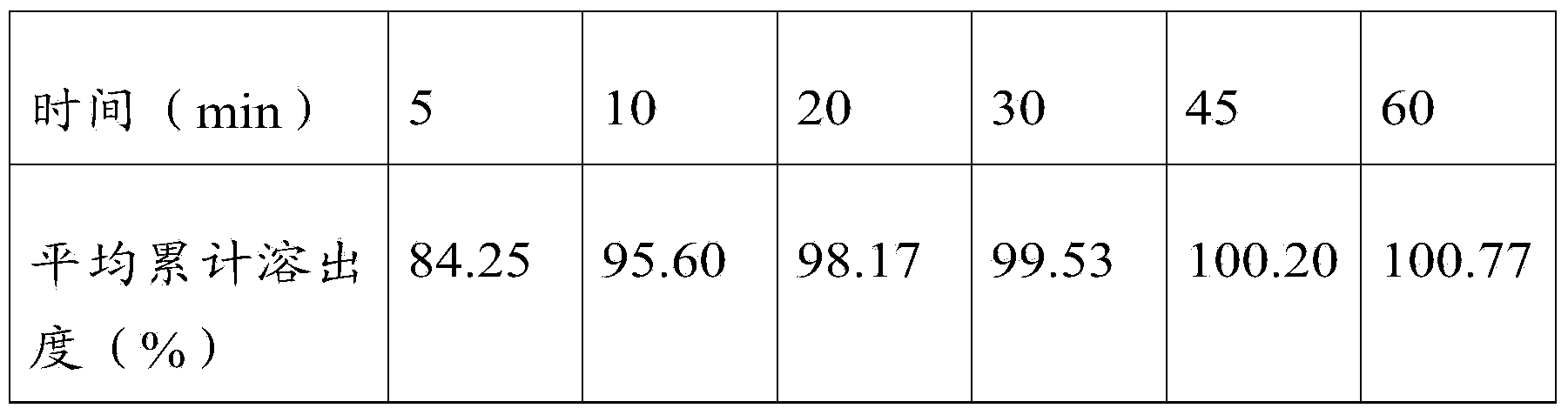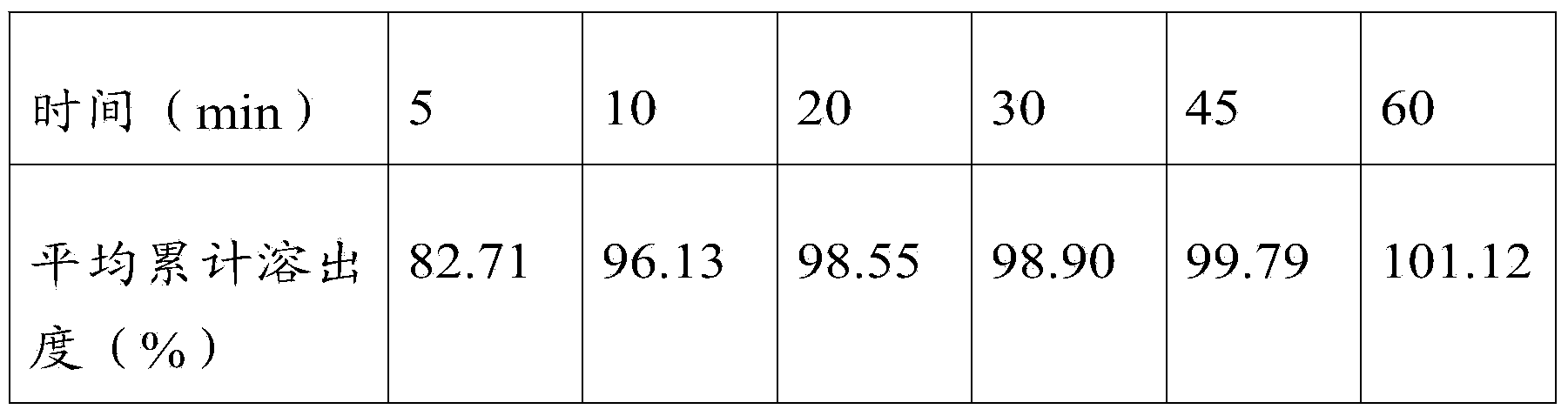Febuxostat tablet and preparation method thereof
A febuxostat and tablet technology, applied in the field of medicine, can solve the problems of high fluidized bed granulation requirements, complex preparation process, and addition of surfactants, etc., to improve bioavailability, easy to obtain auxiliary materials, and promote full disintegration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of febuxostat tablet is also provided for above-mentioned embodiment, comprises:
[0030] Weigh febuxostat, filler, disintegrant, binder, lubricant, wetting agent;
[0031] Blending the febuxostat, the filler, the disintegrating agent, and the binder to obtain a first mixture, and pulverizing;
[0032] Filtering for the first time, the fineness of the first filtering is 80-200 mesh;
[0033] adding a wetting agent to the first mixture after the first filtration;
[0034] Filtration and granulation for the second time to obtain wet granules;
[0035] Dry to obtain dry granules;
[0036] sieving the dry particles;
[0037] adding a disintegrant and a lubricant to the dry granules obtained after the sieving, and blending to obtain a second mixture;
[0038] The second mixture is compressed into tablets according to the set tablet weight, and the compressed tablets are coated.
[0039] During the preparation, febuxostat is mixed with fillers, di...
example 2
[0046] Example 2: In parts by weight, 10 parts of febuxostat, 40 parts of filler, 5 parts of disintegrant, 1 part of binder, 1 part of lubricant, and an appropriate amount of wetting agent.
example 3
[0047] Example 3: In parts by weight, 20 parts of febuxostat, 80 parts of filler, 15 parts of disintegrant, 10 parts of binder, 3 parts of lubricant, and an appropriate amount of wetting agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com