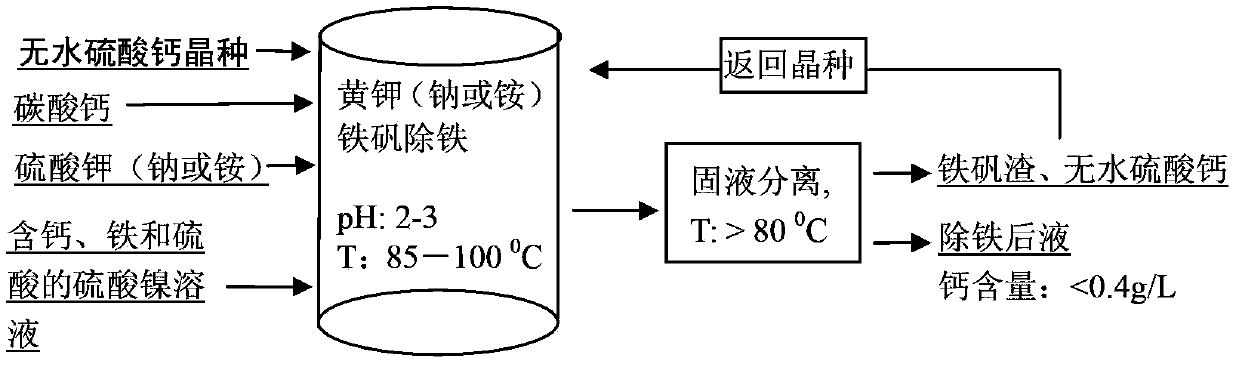
Method for calcium removal during iron precipitation of nickel sulfate solution containing iron and free sulfuric acid
A technology of nickel sulfate and anhydrous calcium sulfate, applied in the field of hydrometallurgy, can solve the problem of high cost, achieve low cost, good calcium removal effect, and avoid the effects of calcium crystallization hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
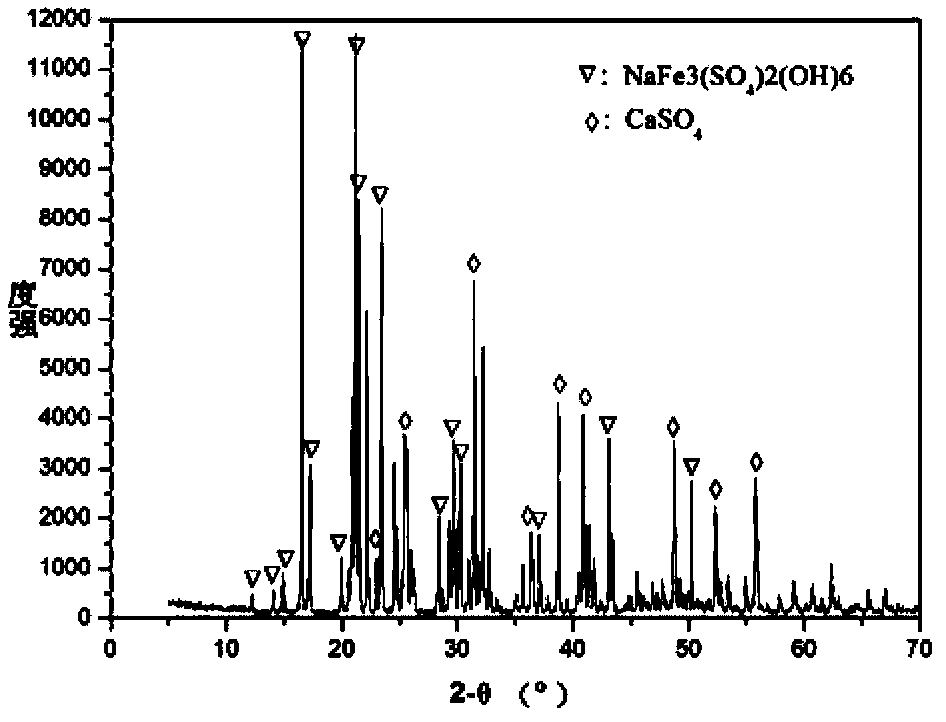
[0026] Get 100 liters of lateritic nickel ore leachate to carry out sinking alum and remove iron, wherein nickel 65 grams per liter, containing Fe 3+ 40 g / L, containing 30 g / L of sulfuric acid, containing 0.6 g / L of calcium, heating the feed liquid to 95°C, adding 1.7 kg of sodium sulfate, adding calcium carbonate powder to adjust the pH value to 2.5-3, and adding anhydrous calcium sulfate at the same time 0.5 kilograms, filter after 3 hours, analysis filtrate calcium is 0.38 gram / liter, and the content of Fe in the filtrate is 0.3 gram / liter, analyzes the composition of filter residue with XRD simultaneously, the result is as follows figure 2 Shown, show that what generate is jarosite and anhydrous calcium sulfate, do not find the generation of dihydrate calcium sulfate; Filtrate is cooled to 40 ℃, do not observe the generation of crystalline calcium sulfate.
Embodiment 2
[0028] Get 100 liters of nickel smelter nickel sulfate waste liquid to neutralize and remove iron, wherein nickel is 65 grams per liter, and Fe 3+10 g / L, containing 30 g / L of sulfuric acid, 0.5 g / L of calcium, heating the feed liquid to 95°C, adding 0.5 kg of sodium sulfate, adding calcium carbonate powder to adjust the pH to 4.5, and adding 0.5 kg of anhydrous calcium sulfate , filter after 3 hours, analysis filtrate calcium is 0.35 gram / liter, and the content of Fe in the filtrate is 0.04 gram / liter; Filtrate is cooled to 40 ℃, does not observe the generation of crystalline calcium sulfate in the process.
Embodiment 3
[0030] Get 100 liters of nickel sulfate feed solution to neutralize and remove iron, wherein nickel is 35 grams per liter, and Fe 3+ 10 g / L, containing 30 g / L of sulfuric acid, containing 0.5 g / L of calcium, heating the feed liquid to 85°C, slowly adding calcium carbonate powder to adjust the pH value to 5, adding 0.5 kg of anhydrous calcium sulfate at the same time, filtering after 3 hours , the analysis filtrate containing calcium is 0.37 grams per liter, and the content of Fe in the filtrate is 0.35 grams per liter; The filtrate is cooled to 40 ℃, does not observe the generation of crystalline calcium sulfate in the process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com