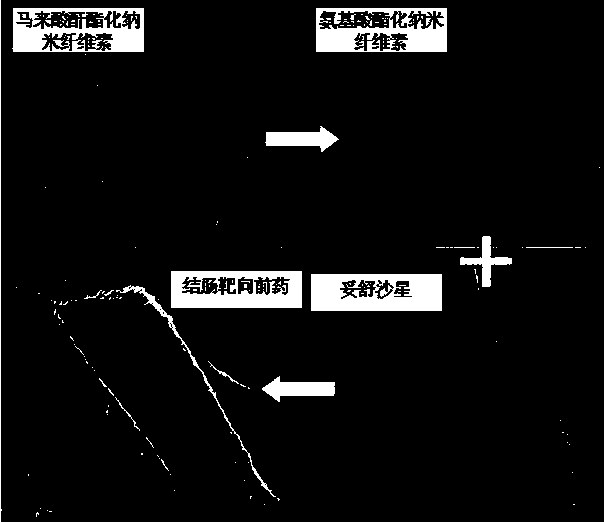
Colon-targeted pro-drug based on nanometer cellulose carrier and preparation method of colon-targeted pro-drug
A technology of nanocellulose and cellulose, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, anti-tumor drugs, etc., can solve the problems of chemical bonds that are difficult to break, have no practical application value, and limit targeting activity, and achieve good results. Effect of Encapsulation, Good Drug Controlled Release Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
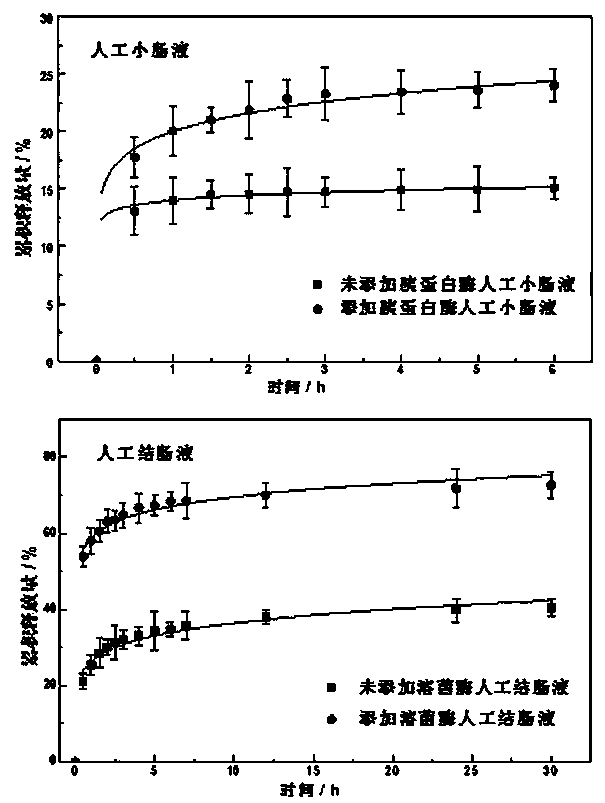
Image
Examples
Embodiment 1
[0020] Weigh 1 g dry sample of nanocellulose, 0.08 g 4-dimethylaminopyridine (DMAP), 1.5 g alanine, 1.25 g 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt Hydrochloride (EDC·HCl), 0.75 g of fluorenylmethoxycarbonyl chloride (Fmoc-Cl), 50 mL of dimethyl sulfoxide was added, the reactants were mixed evenly, and the reaction was performed under magnetic stirring at room temperature for 20 h. After the reaction was completed, the liquid was removed by centrifugation at 9000 rpm, and then the remaining solid sample was washed with deionized water multiple times, and then washed twice with ethanol solution to obtain an Fmoc-protected amino nanocellulose sample. To remove Fmoc protection, disperse it in 20 mL of 10% (v / v) piperidine / DMF solution, stir for 20 min to remove Fmoc, and obtain amino acid esterified nanocellulose, which is vacuum-frozen at -53°C Dry the sample.
[0021] Weigh 0.1 g dry sample of amino acid esterified nanocellulose, 0.3 g N-hydroxysuccinimide (NHS), 0.3 ...
Embodiment 2
[0023]Weigh 1 g dry sample of nanocellulose, 0.08 g 4-dimethylaminopyridine (DMAP), 1.5 g leucine, 1.25 g 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt Hydrochloride (EDC·HCl), 0.75 g of fluorenylmethoxycarbonyl chloride (Fmoc-Cl), 50 mL of dimethyl sulfoxide was added, the reactants were mixed evenly, and the reaction was performed under magnetic stirring at room temperature for 20 h. After the reaction was completed, the liquid was removed by centrifugation at 9000 rpm, and then the remaining solid sample was washed with deionized water multiple times, and then washed twice with ethanol solution to obtain an Fmoc-protected amino nanocellulose sample. To remove Fmoc protection, disperse it in 20 mL of 10% (v / v) piperidine / DMF solution, stir for 20 min to remove Fmoc, and obtain amino acid esterified nanocellulose, which is vacuum-frozen at -53°C Dry the sample.
[0024] Weigh 0.1 g dry sample of amino acid esterified nanocellulose, 0.5 g N-hydroxysuccinimide (NHS), 0.5 g...
Embodiment 3
[0026] The filter paper was disintegrated in a fiber standard disintegrator at 3000 r / min for 20 minutes to obtain a uniformly dispersed filter pulp, which was freeze-dried for later use. Take 3.0 g of dried filter pulp, stir and disperse in 100 mL of acetic acid solution, and let it stand overnight (15 h) to preactivate the cellulose. Then 1.5 mL of concentrated sulfuric acid was added dropwise as a catalyst, and the suspension was placed in an oil bath at 80 °C and stirred at 300 r / min for 6 h. After reacting for a certain period of time, the reaction system was ultrasonically treated at an ultrasonic frequency of 40 kHz, a power of 250 W, an ultrasonic temperature of 75 °C, and an ultrasonic time of 5 h. After the reaction was completed, the suspension was centrifuged repeatedly with deionized water at 9000 r / min, and then unreacted reagents and by-products were eluted with acetone / ethanol mixture (1 / 1 volume ratio). Finally, the collected maleic anhydride esterified nanoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com