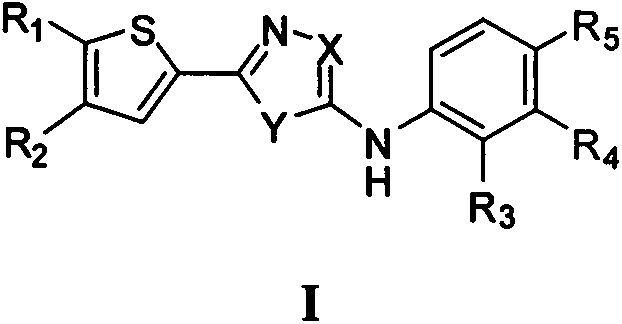
2-imidazole ring-substituted thiophene PLK1 (Polo-like kinase 1) inhibitors and applications thereof
A technology of thiophene and pyrazole, applied in the field of medicinal chemistry, can solve the problems of safety, stability and off-target effects of RNA interference technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
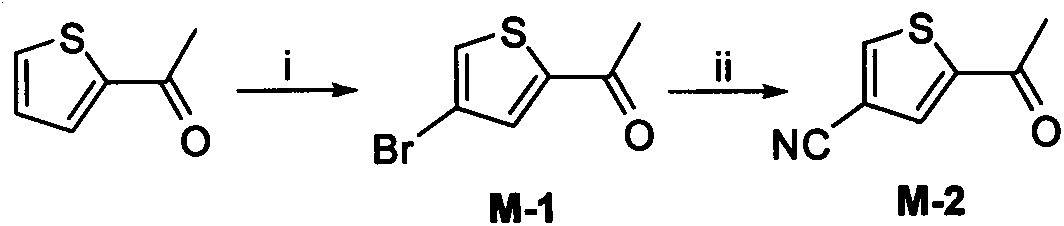
[0132] 2-Acetyl-4-bromothiophene (M-1)
[0133] Add 3.00g (23.81mmol) of 2-acetylthiophene (23.81mmol), 9.53g (71.43mmol) of anhydrous aluminum chloride and 80ml of carbon tetrachloride into a 250ml single-necked flask, cool to -40℃, and slowly add liquid bromine after 10 minutes of stability. 3.81g (23.81mmol) of carbon tetrachloride solution (20ml), drip in 0.5h, and stir at room temperature. After 12h, TLC showed that there was no raw material left. The reaction system was poured into a mixture of saturated NaOH solution and crushed ice, extracted with ethyl acetate (75ml×3), combined the organic phases, washed twice with saturated brine, anhydrous MgSO 4 dry. After concentration under reduced pressure on the column layer (PE:EA=500:1), 2.74 g of light yellow oily liquid was obtained, with a yield of 72.6%.
[0134] 1 HNMR(300MHz DMSO-d 6 )δ: 7.65 (1H, d, ArH), 6.91 (1H, d, ArH), 2.57 (3H, s, -COC H 3 ).MS[M+H] + 205.2, 207.1.
Embodiment 2
[0136] 2-Acetyl-4-cyanothiophene (M-2)
[0137] Add M-10.5g (2.45mmol), cuprous cyanide 0.66g (3.68mmol), potassium iodide 0.04g (0.245mmol) and 10ml anhydrous DMF into a 100ml pressure tube. The temperature was raised to 190°C under inert gas protection, and TLC showed that there was no raw material remaining after 3h. The reaction system was poured into a mixture of ammonia water and crushed ice, extracted with ethyl acetate (20ml×3), combined the organic phases, washed twice with saturated brine, and anhydrous MgSO 4 After drying, concentration under reduced pressure and column chromatography (PE:EA=100:1), 0.23 g of light yellow solid was obtained, with a yield of 62.2%. mp.112~114℃ (document mp.111~113℃).
Embodiment 3
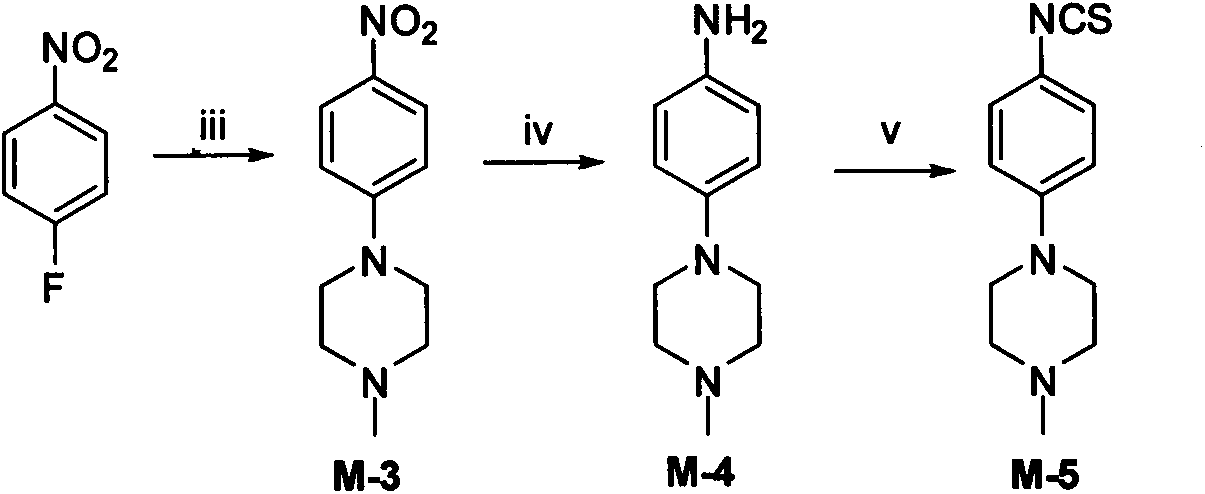
[0139] 1-methyl-4-(4-nitrobenzene)piperidine (M-3)
[0140] Add 1.41g (10.00mmol) of p-fluoronitrobenzene, 1.01g (10.00mmol) of 1-methylpiperazine, 1.01g (10.00mmol) of triethylamine and 25ml of anhydrous DMF into a 50ml single-necked flask, and react at room temperature for 24h Later TLC showed that there was no raw material left. Evaporate the solvent under reduced pressure, add ice water, adjust the pH to 8 with saturated sodium carbonate solution, extract with ethyl acetate (40ml×3), combine the organic phases, wash twice with saturated brine, anhydrous MgSO 4 dry. After concentration under reduced pressure and column chromatography (PE:EA=1:1), 1.38 g of orange-yellow needle-like crystals were obtained, with a yield of 85.3%, mp. 35-38°C.
[0141] MS[M+H] + 222.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com