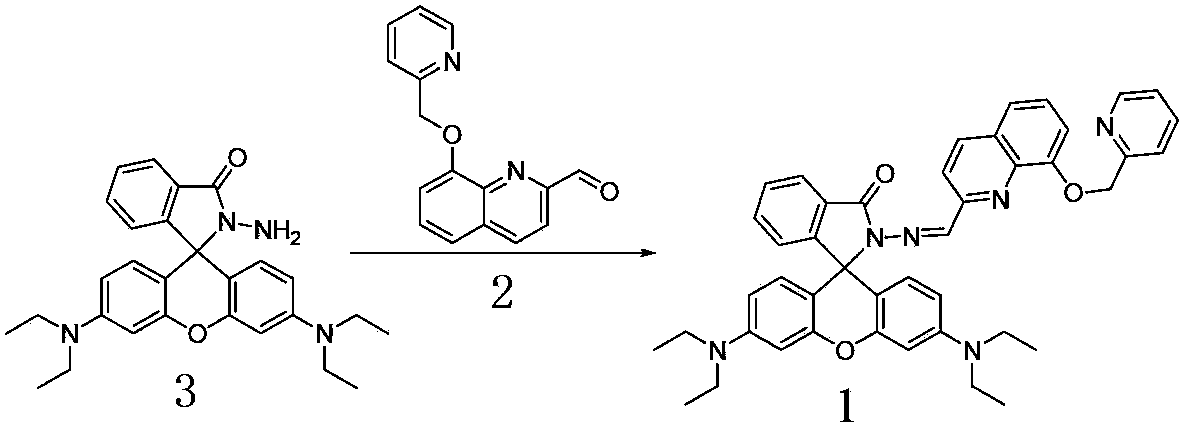
Rhodamine B derivative, its preparation and application
A technology of derivatives and pyridine, which is applied in the field of rhodamine B derivatives and its preparation and application, can solve the problems of coexisting ions affecting detection, long response time, poor water solubility of probes, etc., and achieve low raw material cost, short response time, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of rhodamine B derivatives
[0044] (1) Dissolve 2-chloromethylpyridine and 8-hydroxyquinoline-2-carbaldehyde in the organic solvent acetonitrile at a molar ratio of 1:1, and react under reflux under stirring for 10 hours. After distilling off the solvent, use silica gel column chromatography The intermediate products 8-hydroxyquinoline-2-carbaldehyde and 2-chloromethylpyridine were isolated with a yield of 80wt%.
[0045] (2) Dissolve the obtained intermediate product 8-(pyridine-2-methyleneoxy)-quinoline-2-carbaldehyde and rhodamine B hydrazine in the organic solvent methanol according to the molar ratio of 1:1, and reflux under stirring After reacting for 10 hours, after the solvent was evaporated, the pure rhodamine B derivatives were separated by silica gel column chromatography, and the pure rhodamine B derivatives were obtained by nuclear magnetic resonance (Bruker AVANCE300MHz nuclear magnetic resonance instrument, Bruker Company, Switz...
Embodiment 2
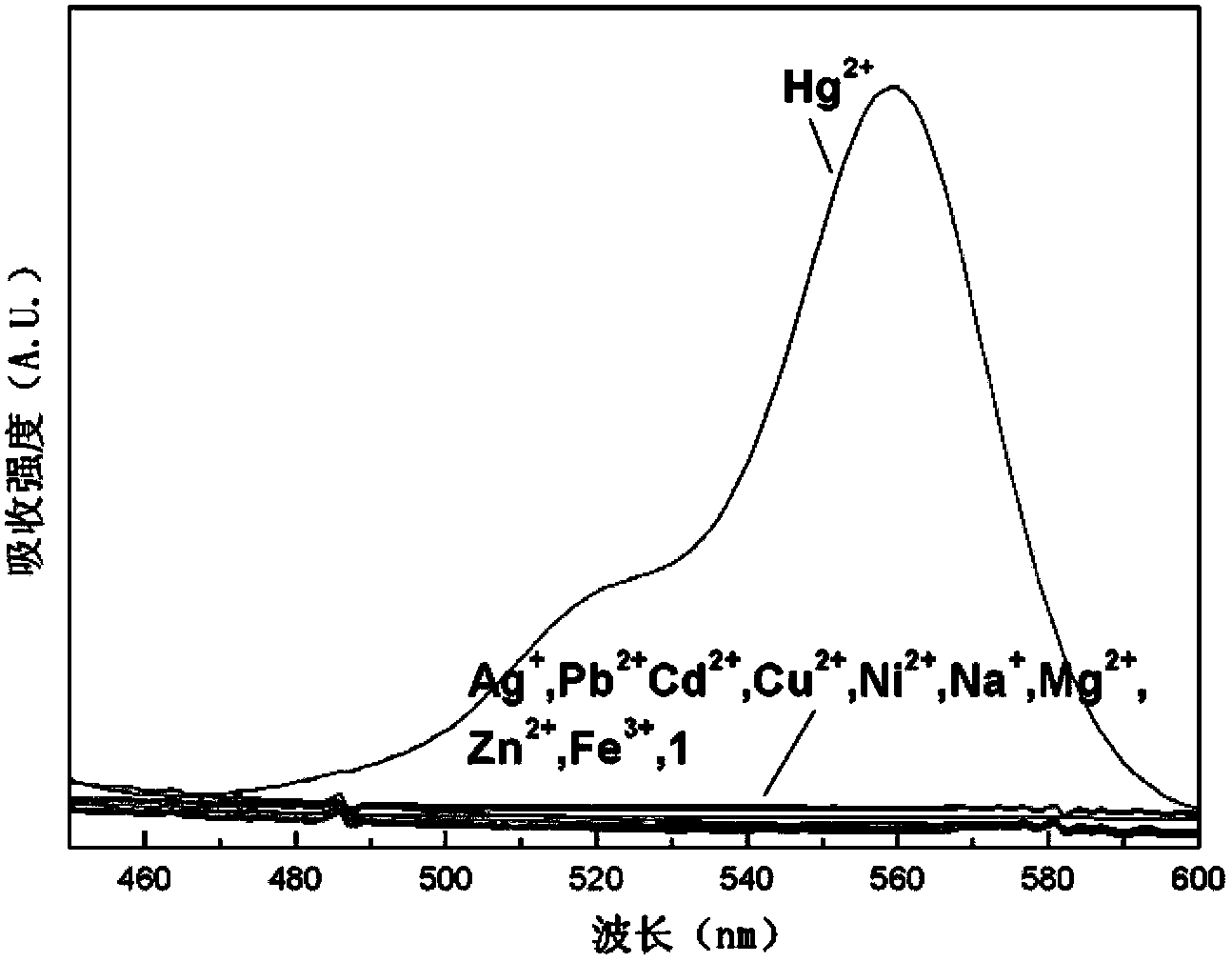
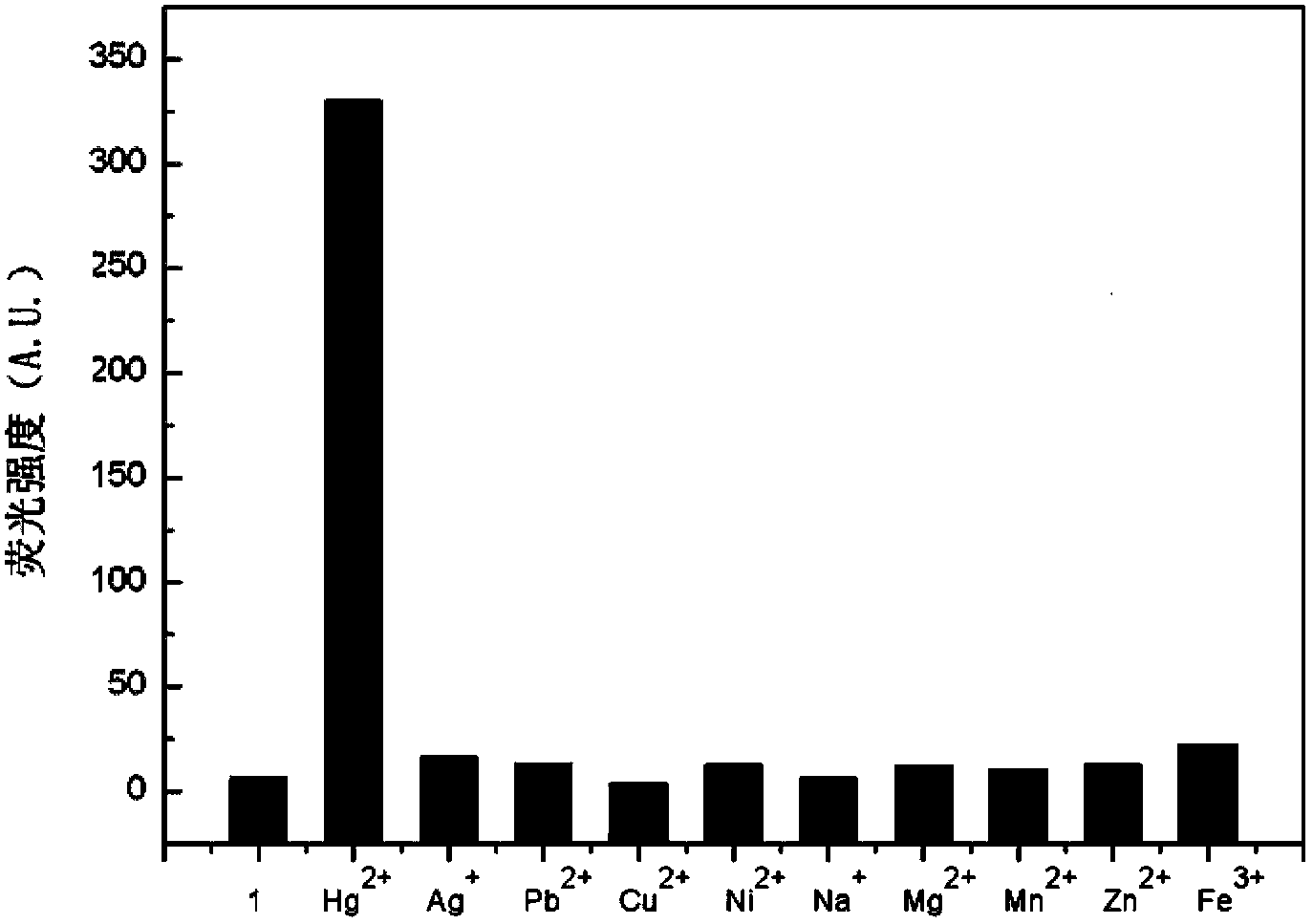
[0052] Example 2: Using rhodamine B derivatives as fluorescent probe molecules to detect mercury ions
[0053] Rhodamine B derivatives were formulated as fluorescent probes into 10 -5 mol / L solution, respectively take 2mL of pure water, tap water, river water and lake water, add the same amount of fluorescent probe solution in turn, react for 1-5 minutes, then detect the fluorescence spectrum, record the fluorescence intensity value at 585nm, experiment It shows that the content of mercury ions in river water and lake water is relatively large, and that in tap water and purified water is relatively low.
[0054] It can be seen from the above experiments that rhodamine B derivatives, as fluorescent probes, have a short response time to mercury ions in water, and have a high response intensity to mercury ions and good selectivity, and can be used for rapid detection of mercury ions in different water sources.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com