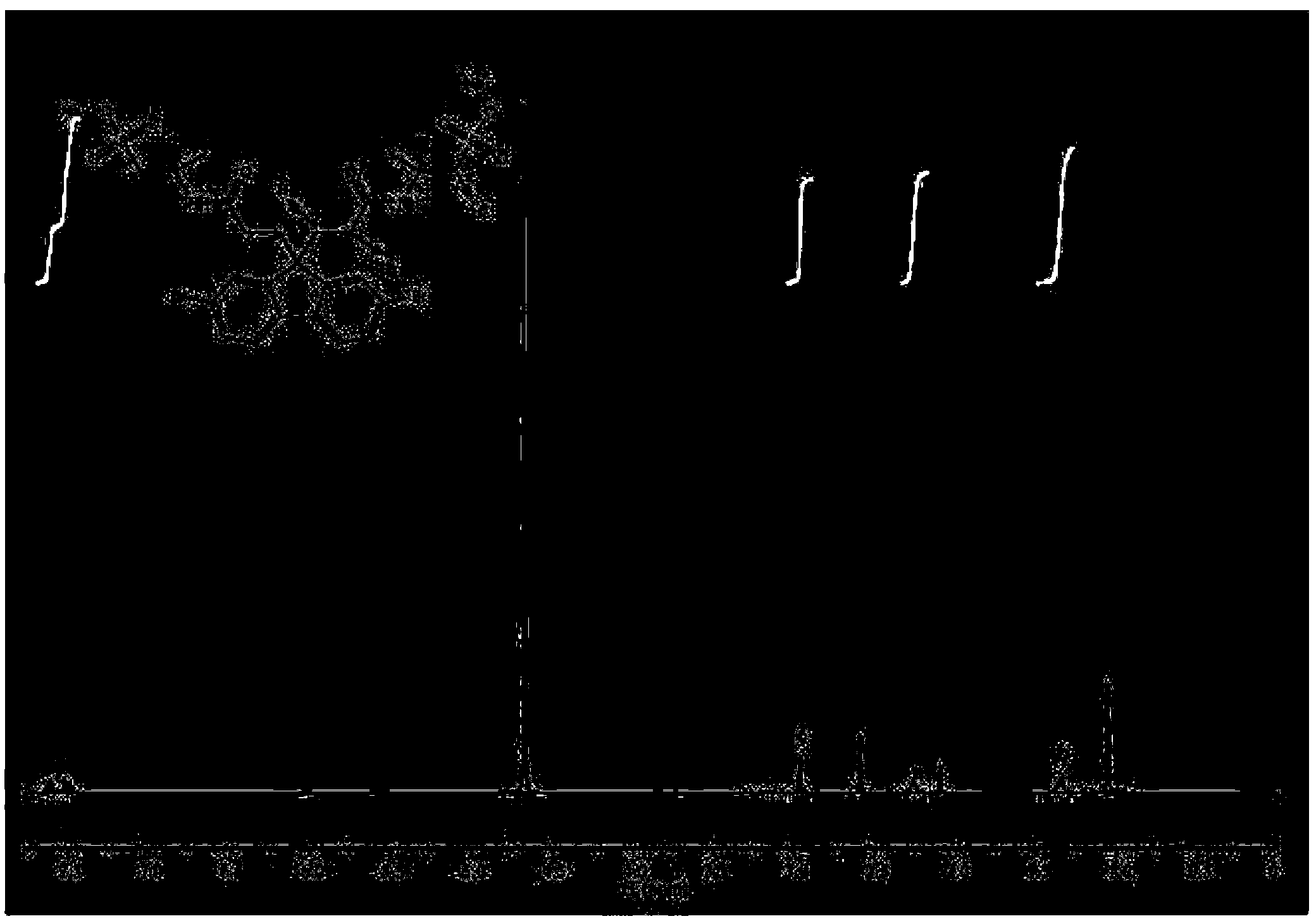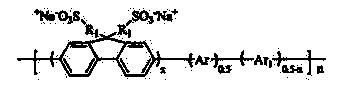Fluorene based sulfonic acid type water/alcohol-soluble conjugated polymer and preparation method thereof
A technology of conjugated polymer and sulfonic acid type, which is applied in the field of fluorene-based sulfonic acid type water/alcohol soluble conjugated polymer and its preparation, can solve the problems of complex synthesis method and high cost, and achieve simple method and short reaction time Short, good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
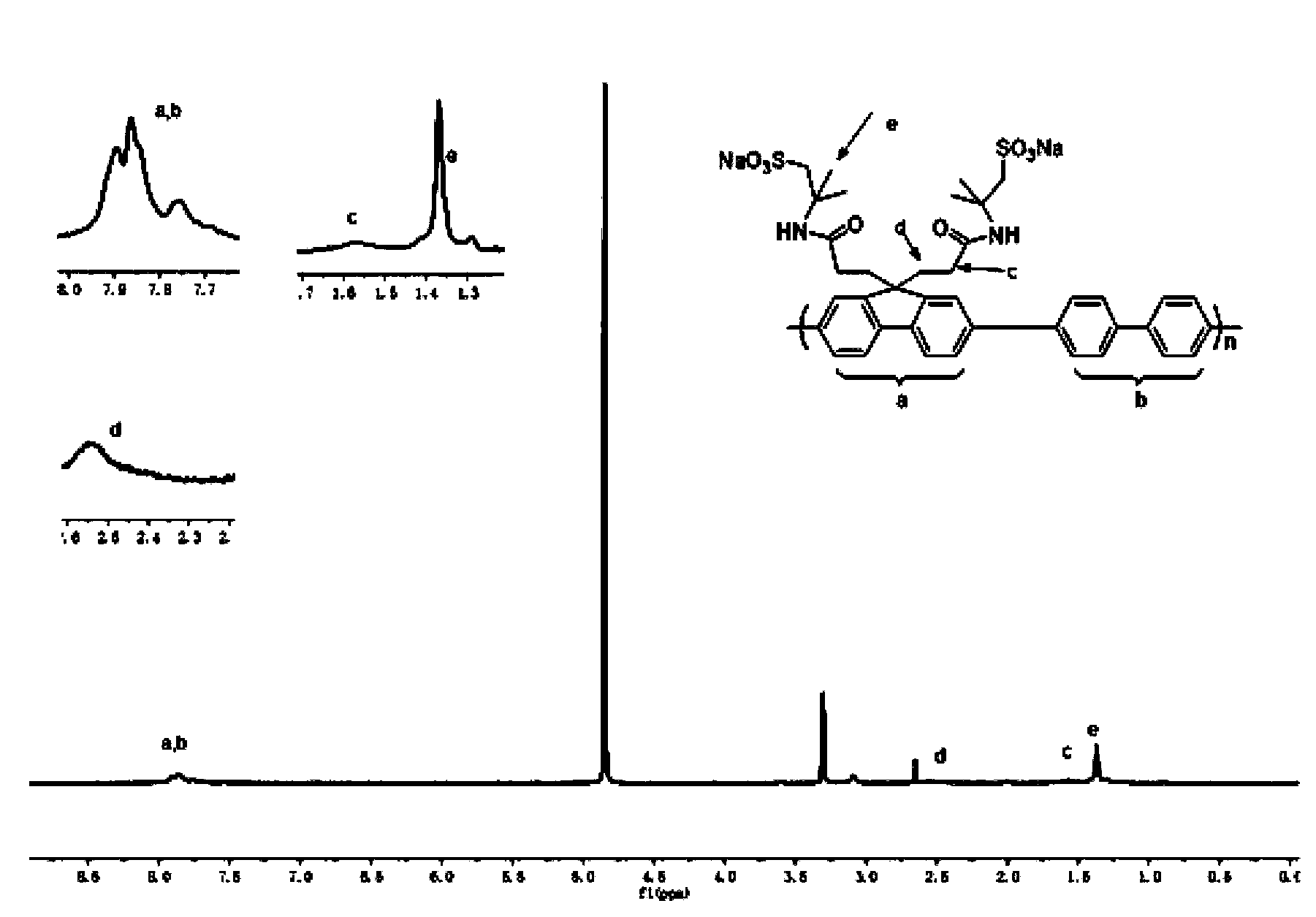
[0041] Preparation of sulfonic acid monomer (2,7-dibromo-9,9-bis-(3-propylamide-2-methylpropanesulfonate sodium)):
[0042]
[0043] In a 250ml four-necked flask equipped with a thermometer and a stir bar, 12.96g (40mmol) of 2,7-dibromofluorene, 0.516g (1.6mmol) of tetrabutylammonium bromide (TBAB) and 25ml of dimethylmethylene were added quantitatively. Sulfone (DMSO), slowly inject 20g of 50% sodium hydroxide solution with a syringe under a nitrogen atmosphere. After heating to 30~40℃ for 1 hour, slowly add 18.23g (0.088mol) dissolved in 40ml DMSO. ) 2-Acrylamide-2-methylpropanesulfonic acid, after dripping, react at 30-40℃ for 10h, and neutralize with hydrochloric acid. Then the solvent was evaporated to dryness by a rotary evaporator, dissolved in ethanol, filtered to remove salt, and most of the solvent was removed by a rotary evaporator, precipitated with ethyl acetate, and dried in a vacuum oven at 70°C to obtain a white powder with a yield of 78.9%.
[0044] Yield %=100%×m...
Embodiment 2
[0047] Preparation of sulfonic acid type conjugated polymer: 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborane-diyl)-9,9-dihexyl Copolymerization of fluorene and 2,7-dibromo-9,9-bis-(3-propylamide-2-methylpropanesulfonate): (PF 6 SO 3 Na)
[0048]
[0049] In a 100ml three-necked flask equipped with a thermometer and a stir bar, 0.3516g (0.6mmol) of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxa Borane-diyl)-9,9-dihexylfluorene, 0.4692g (0.6mmol) 2,7-dibromo-9,9-bis-(3-propylamide-2-) prepared in Example 1 Sodium methyl propanesulfonate), 0.004g (0.018mmol) of Pd (OAC) 2 , 0.026g (0.072mmol) P(C 6 H 12 ) 3 BF 4 , 8ml Et with a mass fraction of 25% 4 Mixed solvent of NOH and 5ml DMSO and 4.5ml toluene. They were mixed uniformly, and the reaction was stopped after reacting at 90°C for 6 hours under a nitrogen atmosphere. Then add 0.0612g (0.5mmol) of phenylboronic acid to react for 2 hours, and finally add 0.078g (0.5mmol) of bromobenzene to react for 2 hours. After the reaction was comple...
Embodiment 3
[0052] 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborane-diyl)-9,9-dioctylfluorene and 2,7-dibromo- Copolymerization of 9,9-bis-(3-propylamide-2-methylpropanesulfonate): (PF 8 SO 3 Na)
[0053]
[0054] In a 100ml three-necked flask equipped with a thermometer and a stir bar, 0.3852g (0.6mmol) of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxa Borane-diyl)-9,9-dioctylfluorene, 0.4692g (0.6mmol) 2,7-dibromo-9,9-bis-(3-propylamide-2-methylpropanesulfonic acid) Sodium), 0.004g (0.018mmol) of Pd (OAC) 2 , 0.022g (0.20mmol) DABCO, 8ml mass fraction is 25% Et 4 Mixed solvent of NOH and 6ml DMSO and 4ml toluene. They were mixed uniformly, and the reaction was stopped after reacting at 90°C for 7 hours under a nitrogen atmosphere. Then add 0.0612g (0.5mmol) of phenylboronic acid to react for 2 hours, and finally add 0.078g (0.5mmol) of bromobenzene to react for 2 hours. After the toluene and water were removed by a rotary evaporator, it was precipitated with ethyl acetate twice, and dried in a va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com