Combined osmotic pump preparation and preparation method thereof
An osmotic pump and combined technology, which is applied in the field of combined osmotic pump preparations and its preparation, can solve problems such as difficult product quality control, residues, and large drug residues, achieve flexible and diverse drug delivery methods, reduce production requirements, and reduce drug production. small residual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
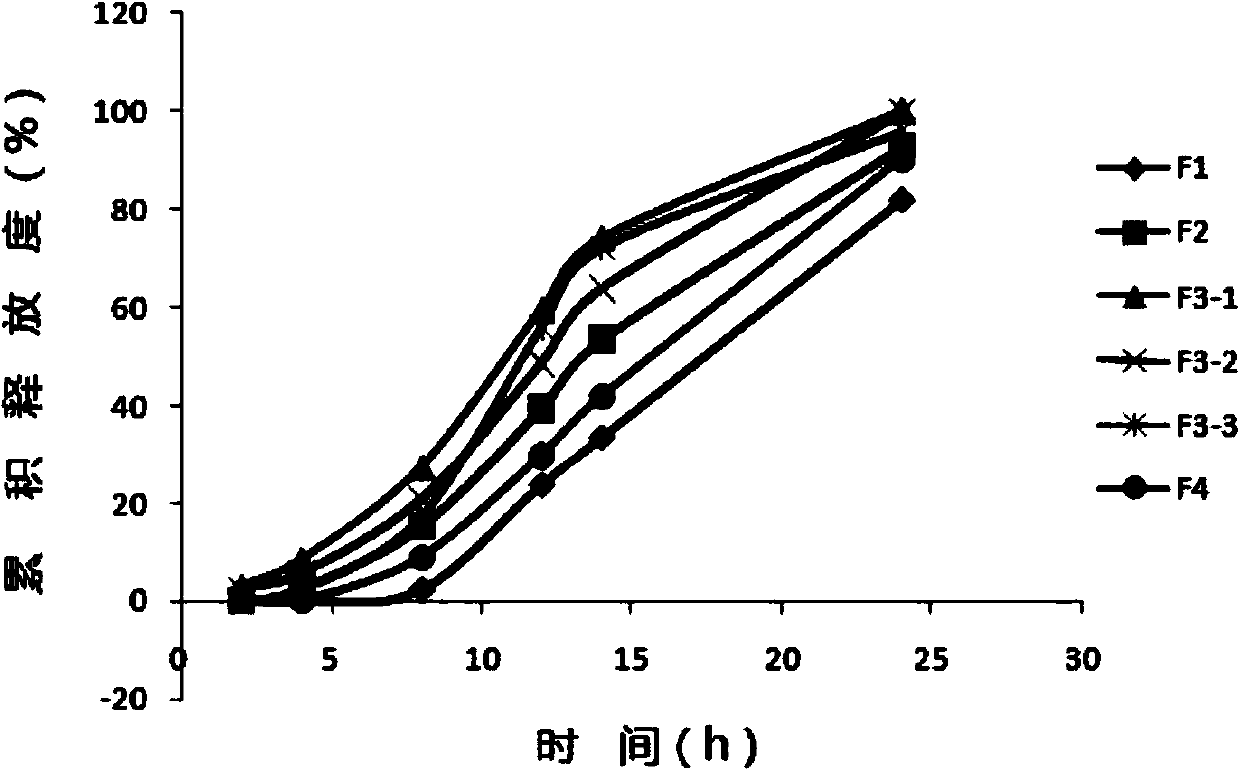
[0062] Example 1 Pariperidone Controlled-Release Capsules
[0063]
[0064]
[0065] Preparation Process
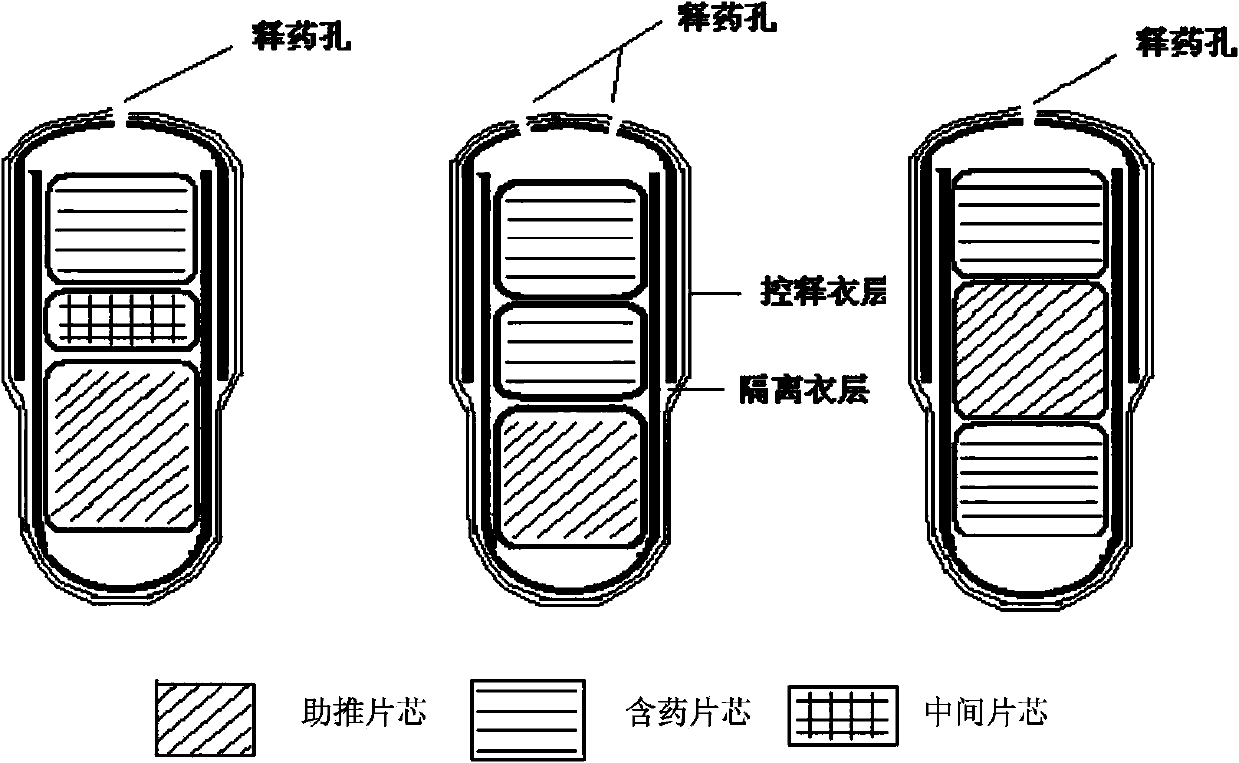
[0066] 1. Chip
[0067] 1. Drug-containing tablet core (1) / drug-containing tablet core (2)
[0068] (1) Pretreatment of raw and auxiliary materials: pass the raw material medicine through a 100-mesh sieve, and pulverize the sucrose through an 80-mesh sieve, for subsequent use;
[0069] (2) Mixing: Weigh 3000 pieces of raw and auxiliary materials in the recipe, and mix them evenly in a wet granulator;
[0070] (3) Granulation: The peristaltic pump feeding speed is set to 15 rpm, and the atomization pressure is 0.1 bar. Spray in the right amount of adhesive to make granules with moderate humidity.
[0071] (4) Drying: place the wet granules in a fluidized bed to dry at 45°C for 1 hour;
[0072] (5) Granulation: pass the dry granules through a 30-mesh stainless steel screen to granulate;
[0073] (6) total mixing: adding magnesium stearate, placing in a three-d...
Embodiment 2
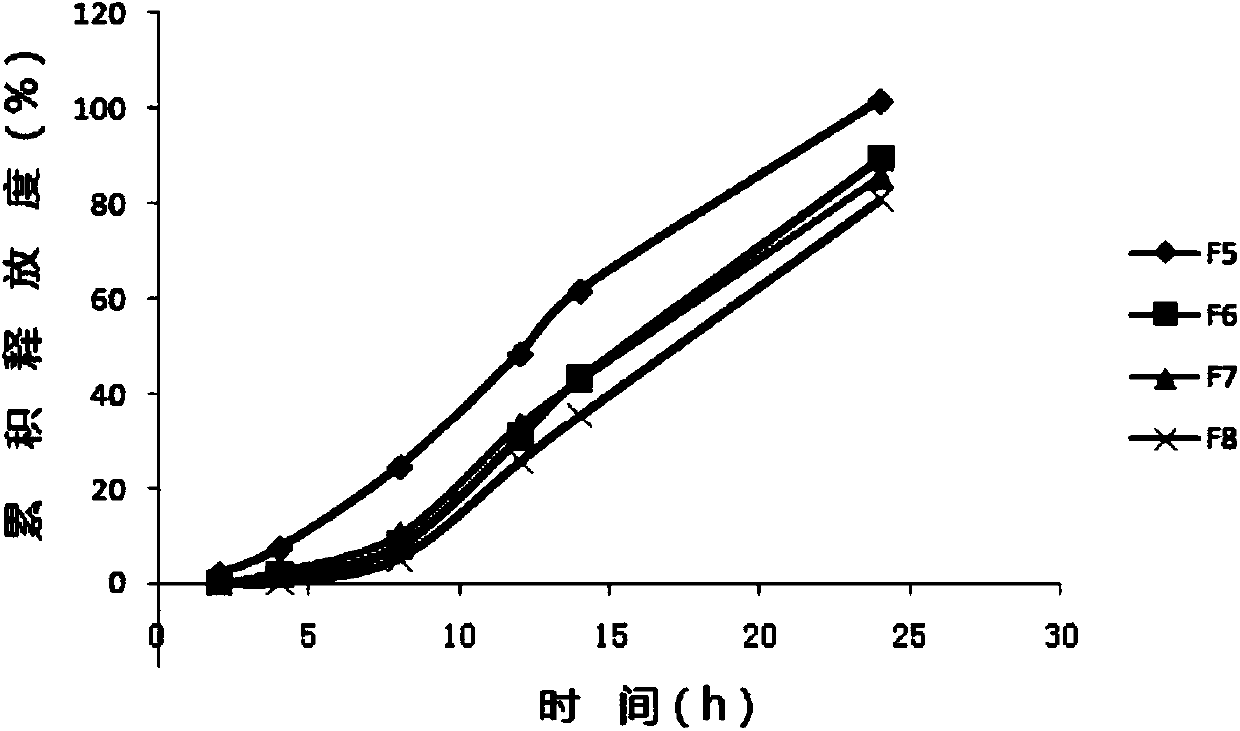
[0101] Embodiment 2 Pariperidone controlled-release capsules
[0102]
[0103]
[0104] Preparation Process
[0105] 1. Chip
[0106] 1. Drug-containing tablet core (1) / drug-containing tablet core (2)
[0107] (1) Pretreatment of raw and auxiliary materials: pass the raw material medicine through a 100-mesh sieve, and pulverize the sucrose through an 80-mesh sieve, for subsequent use;
[0108] (2) Mixing: Weigh 3000 pieces of raw and auxiliary materials in the recipe, and mix them evenly in a wet granulator;
[0109] (3) Granulation: The peristaltic pump feeding speed is set to 10 rpm, and the atomization pressure is 0.1 bar. Spray in the right amount of adhesive to make granules with moderate humidity.
[0110] (4) Drying: place the wet granules in a fluidized bed to dry at 45°C for 1 hour;
[0111] (5) Granulation: pass the dry granules through a 30-mesh stainless steel screen to granulate;
[0112] (6) total mixing: adding magnesium stearate, placing in a thre...
Embodiment 3
[0141] Embodiment 3 Pariperidone controlled-release capsules
[0142]
[0143]
[0144] Preparation Process
[0145] 1. Chip
[0146] 1. Drug-containing tablet core (1) / drug-containing tablet core (2)
[0147] (1) Pretreatment of raw and auxiliary materials: pass the raw material medicine through a 100-mesh sieve, and pulverize the sodium chloride through an 80-mesh sieve, for subsequent use;
[0148] (2) Mixing: Weigh 3000 pieces of raw and auxiliary materials in the recipe, and mix them evenly in a wet granulator;
[0149] (3) Granulation: The peristaltic pump feeding speed is set to 15 rpm, and the atomization pressure is 0.1 bar. Spray in the right amount of adhesive to make granules with moderate humidity.
[0150] (4) Drying: place the wet granules in a fluidized bed to dry at 45°C for 1 hour;
[0151] (5) Granulation: pass the dry granules through a 30-mesh stainless steel screen to granulate;
[0152] (6) total mixing: adding magnesium stearate, placing i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com