Novel freeze-drying protective additive for duck virus hepatitis live vaccines
A technology of duck viral hepatitis and freeze-drying protective agent, which is applied to antiviral agents, freeze-dried delivery, and medical preparations of non-active ingredients, etc., which can solve the problems of large loss of antigenic activity, difficulties in vaccine storage and transportation, and thermal stability of vaccines Sexuality and antigenic activity protection ability is not strong
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] ——Preparation of antigen for duck viral hepatitis live vaccine (A66 strain)
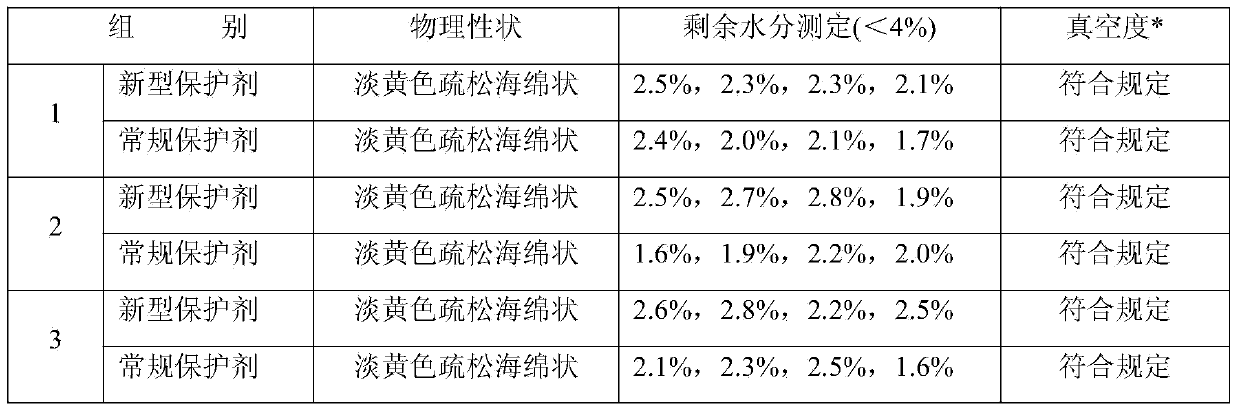
[0036] Take the virus seed of duck hepatitis virus A66 strain used for production and dilute it 100 times with sterilized physiological saline, inoculate the allantoic cavity of well-developed 9-10-day-old SPF chicken embryos, 0.2ml per embryo, seal the holes, and place at 36-37°C Continue to incubate, and discard the chicken embryos that died before 36 hours. After 36 hours, light the eggs every 4-8 hours, take out the dead chicken embryos within 72 hours at any time, and cool them at 4-8°C. Take out the chicken embryos that have been cooled for 4 to 24 hours, disinfect the air chamber with tincture of iodine, remove the eggshells with aseptic surgery, and collect the chorioallantoic membrane, embryo fluid (allantoic fluid and amniotic fluid) and fetuses from diseased chicken embryos. Chicken embryos were mixed into one group, homogenized at high speed to make a suspension, placed in a steri...
Embodiment 2
[0038] ——Preparation of protective agent and preparation of vaccine (1)
[0039] The new lyoprotectant consists of the following components in terms of weight percentage: trehalose 2, glucose 1%, glycine 3%, D-sorbitol 1%, polyvinylpyrrolidone (PVP-k30) 1%, gelatin 0.8%, the balance For injection water. Dissolve the above substances completely, and sterilize by autoclaving at 116°C for 30 minutes.
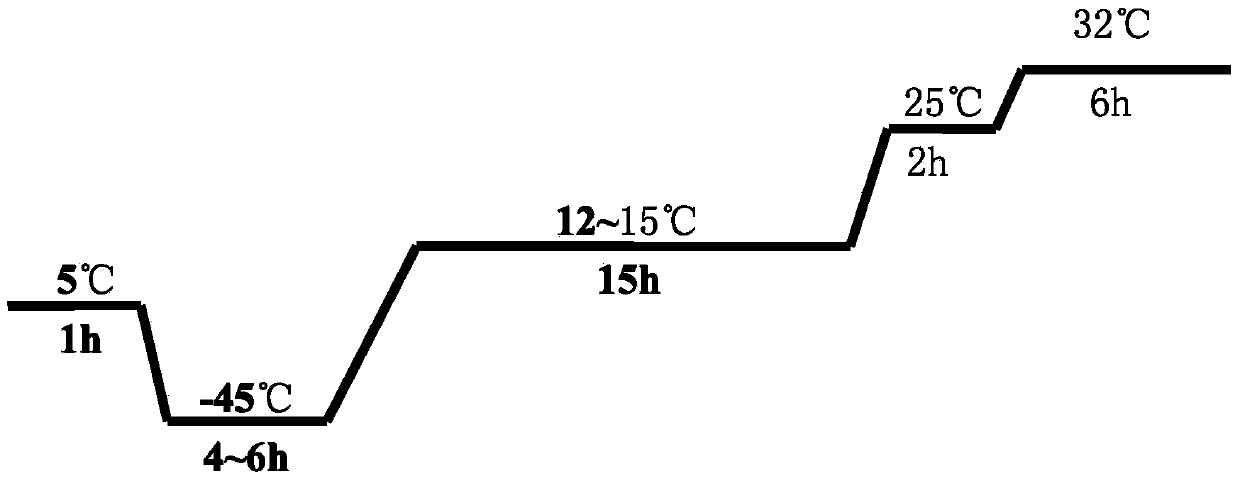
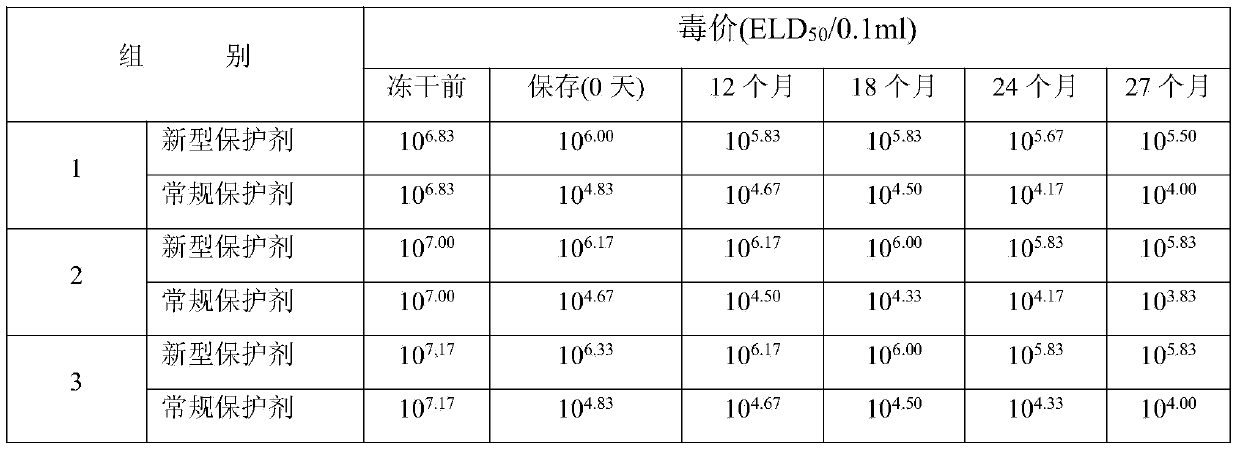
[0040] Take the new lyoprotectant and the 5% sucrose skimmed milk protectant used for the control, mix them with the qualified duck viral hepatitis antigen solution at a volume ratio of 1:1, and pack them into 7ml vials, 2ml per bottle , half stoppered with butyl rubber three-pronged plug, put it into a freeze dryer box pre-cooled to 5°C, lower the temperature to -45°C at a speed of 0.5°C / min, keep the vaccine for 4 hours to freeze the vaccine, and vacuum to 5Pa Then, raise the temperature to 15°C at a rate of 1°C / min and maintain at this temperature for 12 hours, then raise the ...
Embodiment 3
[0042] ——Preparation of protective agent and preparation of vaccine (2)
[0043] The new lyoprotectant consists of the following components in terms of weight percentage: trehalose 3.5%, glucose 2%, glycine 5.5%, D-sorbitol 2%, polyvinylpyrrolidone (PVP-k30) 2%, gelatin 1%, plus Water for injection to 100%. Dissolve the above substances completely, and sterilize by autoclaving at 116°C for 30 minutes.
[0044] Take the new lyoprotectant and the 5% sucrose skimmed milk protectant used for the control, mix them with the qualified duck viral hepatitis antigen solution at a volume ratio of 1:1, and pack them into 7ml vials, 3ml per bottle , half-stopped with butyl rubber three-pronged plug, put it into a freeze dryer box pre-cooled to 5°C, lower the temperature to -45°C at a rate of 0.75°C / min, keep it for 5 hours to freeze the vaccine, and then vacuum to 5Pa Afterwards, raise the temperature to 15°C at a rate of 1°C / min, maintain at this temperature for 13.5 hours, then raise t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com