Separation method for epichlorohydrin
A technology of epichlorohydrin and separation method, which is applied in the field of separation of epichlorohydrin, can solve the problem of high energy consumption, and achieve the effect of reducing energy consumption and good phase separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
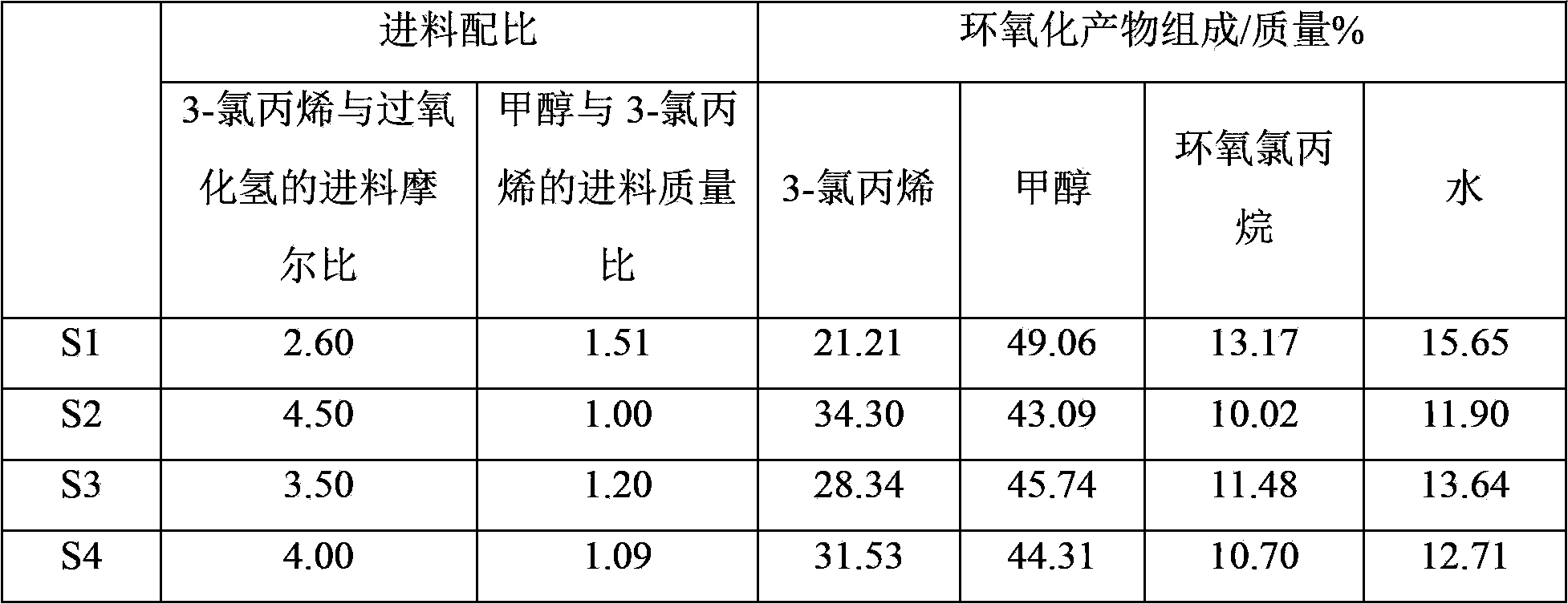
[0054] At 24°C, 100 parts by mass of the known solution S1 containing epichlorohydrin, methanol, 3-chloropropene and water was first mixed with 50 parts by mass of water (the density at 20°C was 998.2 kg / m3, The boiling point is 100°C) contacting and mixing, after standing for stratification, collect the heavy phase of the lower layer, and then use 150 parts by mass of p-chloroanisole (TSI (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1160 kg / m3, the boiling point is 200°C) divided into five parts of equal mass and extracted the upper light phase five times, combined all the heavy phases to obtain the p-chloroanisole phase, epichlorohydrin, methanol in the p-chloroanisole phase , 3-chloropropene and water content are respectively 6.97 mass %, 1.24 mass %, 11.36 mass % and 0.21 mass %, the light phase obtained at last is the wat...
Embodiment 2
[0068] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
[0069] At 12°C, 100 parts by mass of the known solution S2 containing epichlorohydrin, methanol, 3-chloropropene and water was first mixed with 60 parts by mass of water (the density at 20°C was 998.2 kg / m3, The boiling point is 100°C) contacting and mixing, after standing to separate layers, collect the lower heavy phase, and then use 120 parts by mass of butyl chloroacetate (Ticier (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1070.4 kg / cubic meter, the boiling point is 183 ℃) divided into five parts of equal mass and extracted the upper light phase five times, combined all the heavy phases to obtain the butyl chloroacetate phase, in the butyl chloroacetate phase, epichlorohydrin, methanol, 3- The contents of allyl chloride and water are respectively 5.96 mass %, 2.14 mass %, 20.68 mass % and 0.46 mass %, and the light phas...
Embodiment 3
[0081] This embodiment is used to illustrate the separation method of epichlorohydrin provided by the present invention.
[0082] At 13°C, 100 parts by mass of the known solution S3 containing epichlorohydrin, methanol, 3-chloropropene and water was first mixed with 60 parts by mass of water (the density at 20°C was 998.2 kg / m3, The boiling point is 100°C) contacting and mixing, after standing for stratification, collect the heavy phase of the lower layer, and then use 100 parts by mass of p-chloroanisole (TSI (Shanghai) Chemical Industry Development Co., Ltd., the density at 20°C is 1160 kilogram / cubic meter, boiling point is 200 ℃) is divided into five parts of equal mass and extracts the light phase of the upper layer five times, and combines all the heavy phases to obtain the p-chloroanisole phase (that is, the organic solvent phase described in the present invention, and the same below ), the contents of epichlorohydrin, methanol, 3-chloropropene and water in the p-chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com