Mono-chloride-dimethylsulfoxide-6-hydroxide radical oxidation iso-aporphine platinum (II) and synthesizing method and application thereof
A technology of dimethyl sulfoxide and hydroxyl oxidation, which is applied in the field of medicine, can solve problems such as synthetic methods and applications that have not yet been seen, and achieve significant in vitro anti-tumor activity and good medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Weigh 6-hydroxylated isoaporphine and dichlorobis(dimethylsulfoxide) platinum (II) in the same amount, 1 mmol each, and dissolve 6-hydroxylated isoaporphine in 50 mL of 95 % (volume) in methanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 20mL of water, the two solutions were mixed, 2mL dimethylsulfoxide was added to the mixed solution, at 70°C After reacting for 24 hours, concentrate and evaporate to remove most of the solvent (80% of the solvent addition), cool to room temperature, stand still, and separate out a reddish-brown solid, separate the solid, and dry to obtain a reddish-brown solid product (90% yield) .
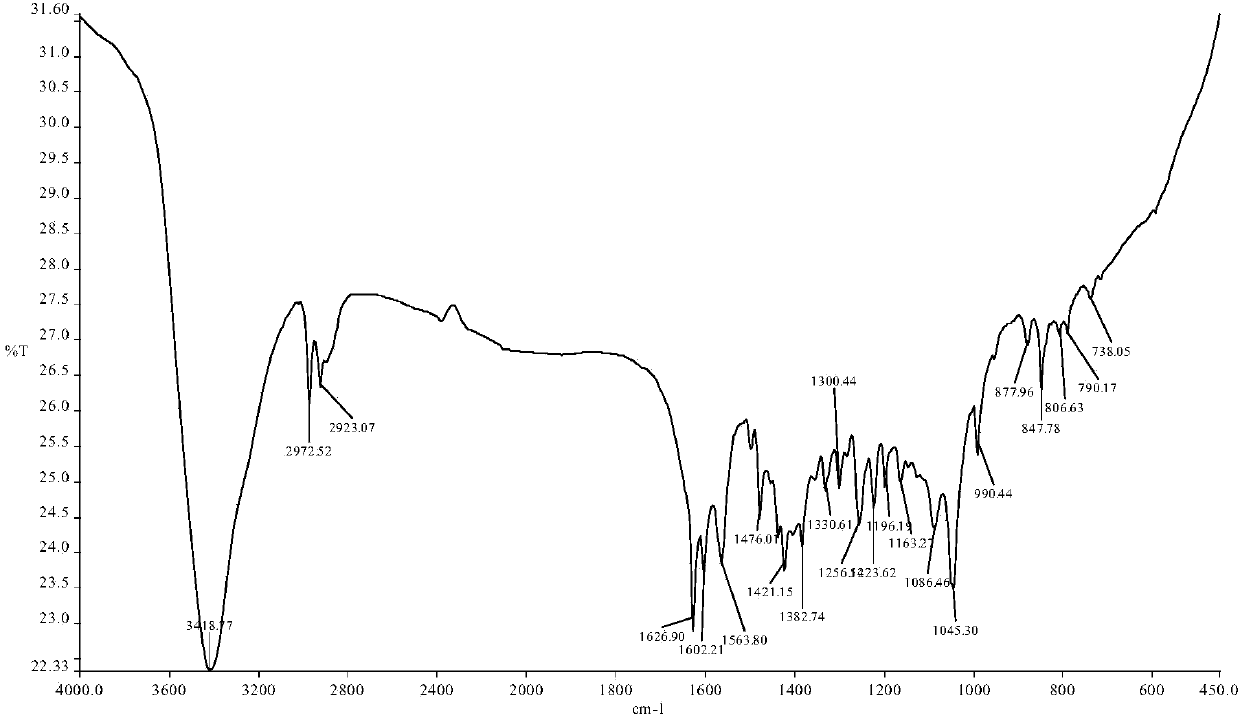
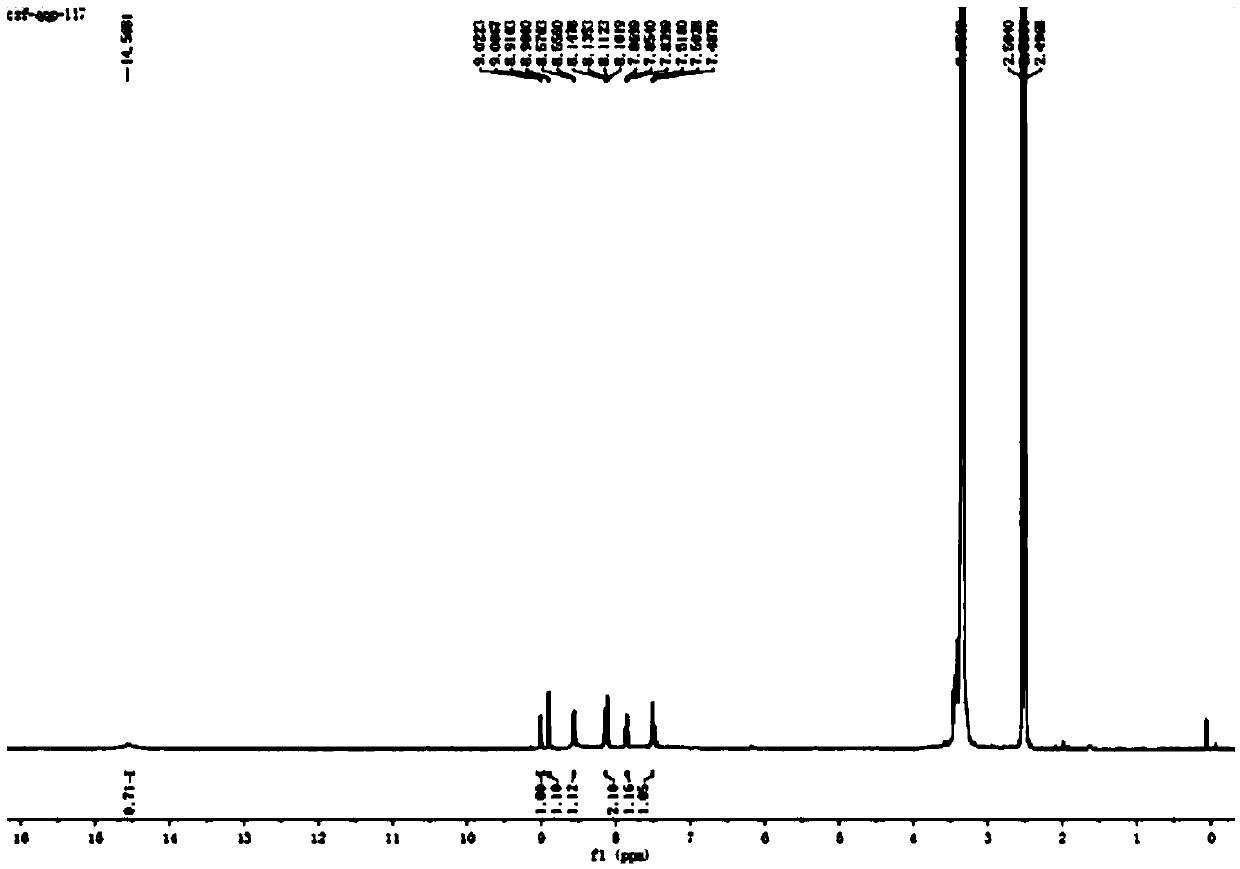
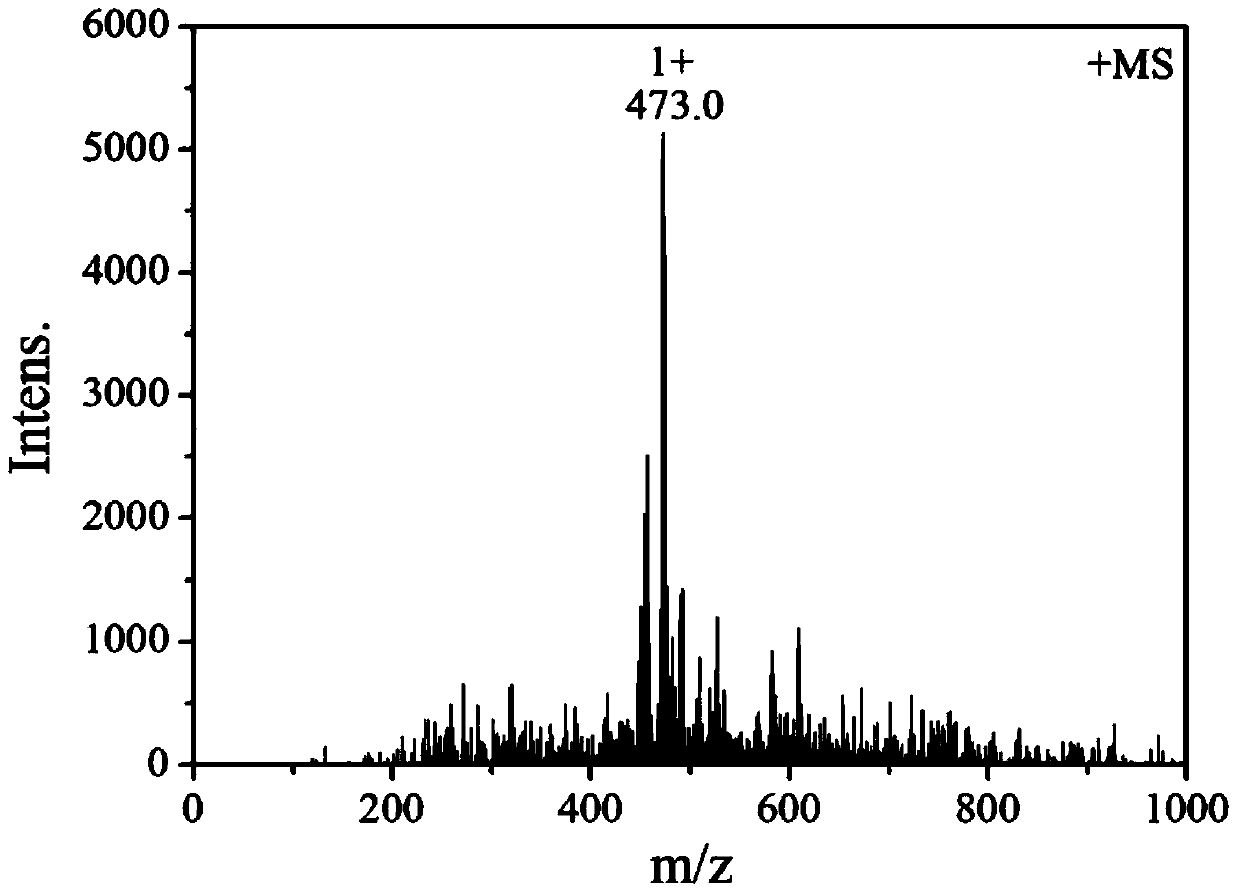
[0036] The resulting reddish-brown solid product was analyzed by infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, electrospray mass spectroscopy, single crystal diffraction and ultraviolet spectroscopy. The specific spectral characteristics are as follows:
[0037] (1) Infrared spectrum, its spectrogram is as foll...
Embodiment 2
[0048] Weigh the same amount of 6-hydroxyisoaporphine and dichlorobis(dimethylsulfoxide) platinum (II), each 1mmol, dissolve 6-hydroxyisoaporphine in 100mL of 70 % (volume) ethanol, dichlorobis(dimethylsulfoxide) platinum (II) was dissolved in 50mL of 40% (volume) methanol, the two solutions were mixed, and the resulting mixed solution was reacted at 60°C for 4 Hours, concentrated and evaporated to remove most of the solvent (85% of solvent addition), cooled to room temperature, left standstill, a reddish-brown solid was separated out, and dried to obtain a reddish-brown solid product with a yield of 60%.
Embodiment 3
[0050] Weigh 6-hydroxylated isoaporphine and dichlorobis(dimethylsulfoxide) platinum (II) in the same amount, 1 mmol each, and dissolve 6-hydroxylated isoaporphine in 50 mL of 95 % (volume) methanol and 95% (volume) ethanol (the volume ratio of methanol and ethanol is 1:1), dissolve dichlorobis(dimethylsulfoxide) platinum (II) in 20mL In the mixed solution of water and methanol (the volume ratio of water and methanol is 1:5), mix the two solutions, add 2mL dimethyl sulfoxide to the mixed solution, react at 80°C for 48 hours, concentrate and evaporate to remove most of the solvent (95% of the amount of solvent added), cooled to room temperature and left to stand, a reddish-brown solid was precipitated, and the solid was separated and dried to obtain monochlorodimethylsulfoxide 6-hydroxyloxyisoapomorphine platinum (II ), the yield was 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 value | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com