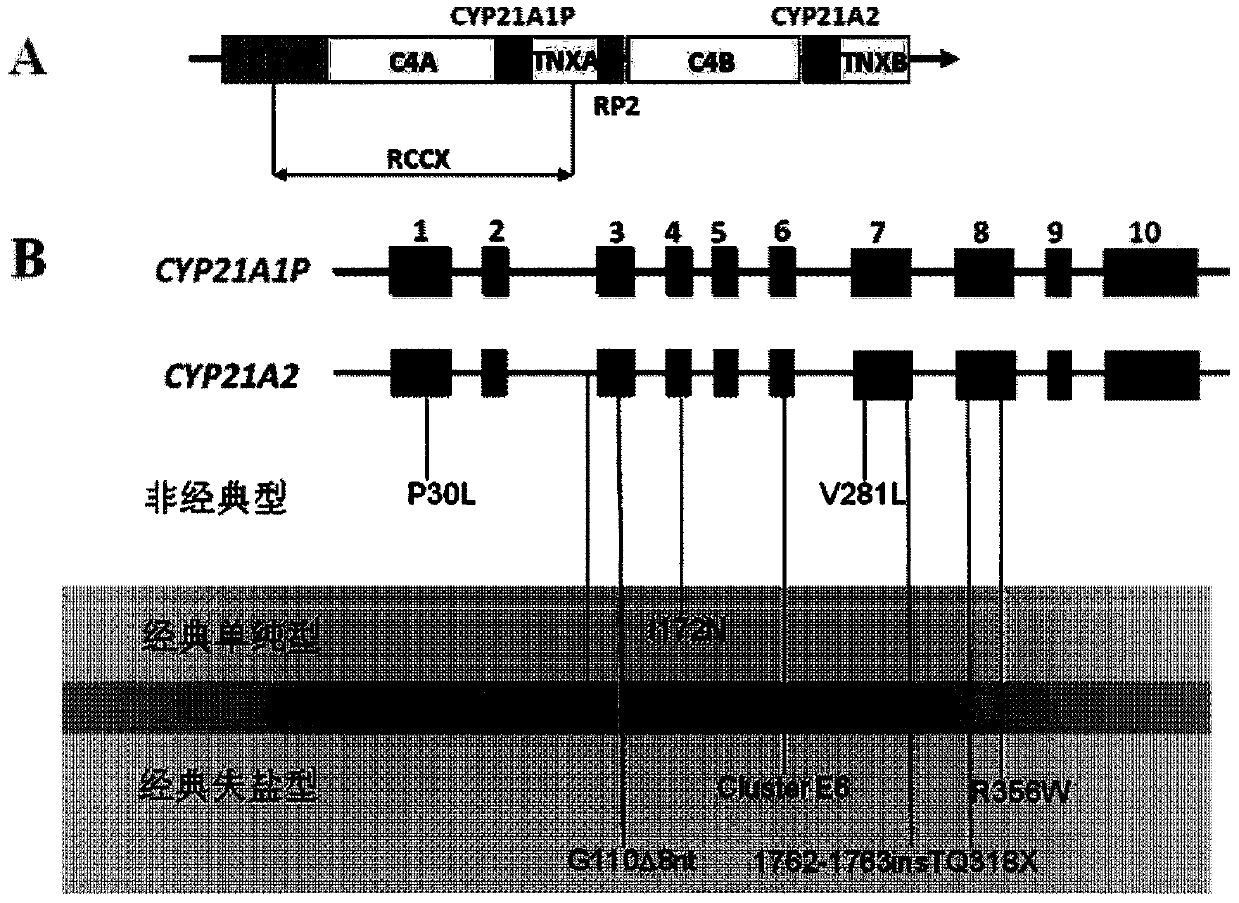
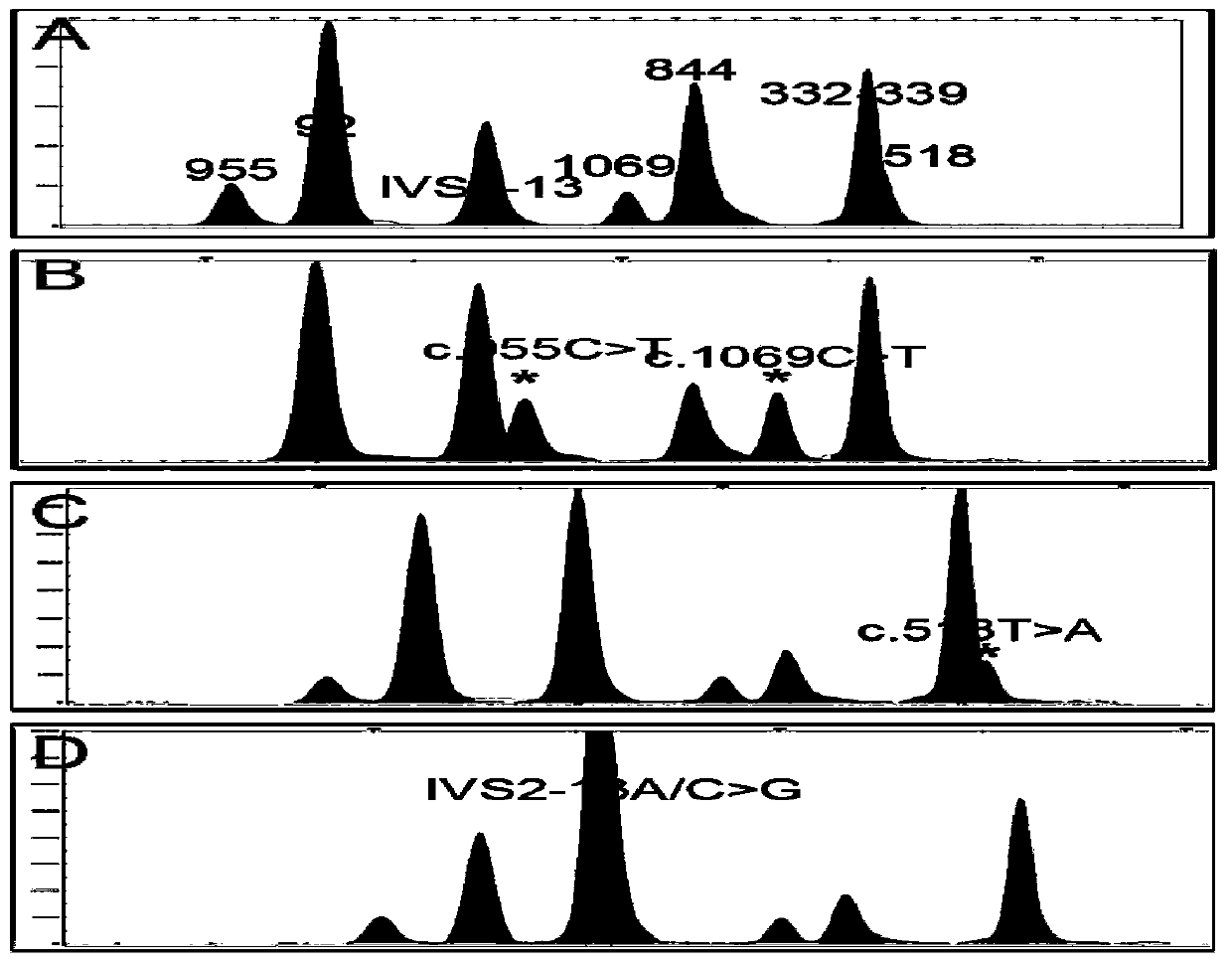
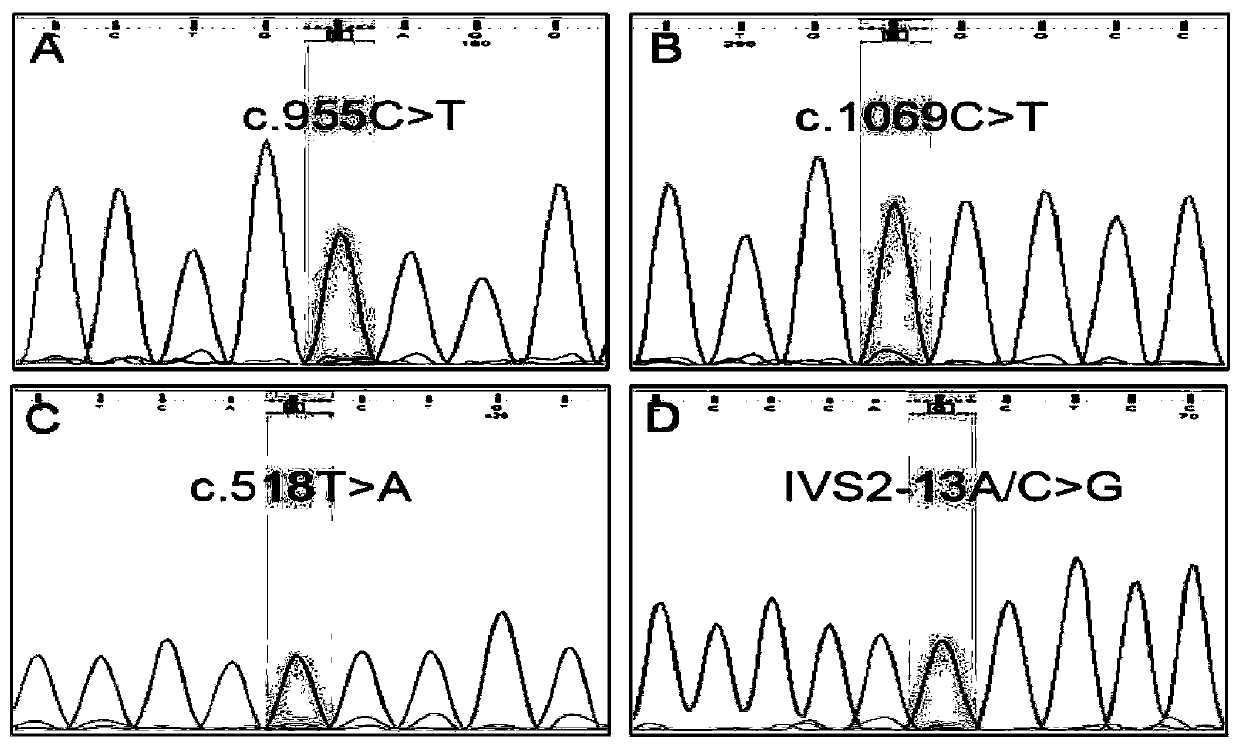
Kit used for screening CYP21A2 gene of Chinese population
A technology of CYP21A2 and reagent kit, which is applied in the direction of microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problems of instability and inability to apply clinically, and achieve the effect of saving detection time and improving diagnostic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1, detection of gene mutation hotspots in CYP21A2 patients
[0050] Sample selection: The samples came from the adrenal hyperplasia detection DNA library of the Reproductive and Genetic Center of Suzhou Municipal Hospital. All patients in the library signed the informed consent form before collection, and agreed to cooperate with follow-up diagnosis, treatment and follow-up.
[0051] In step 1, a nested PCR amplification reaction is performed for two segments of the CYP21A2 gene.
[0052] Primary amplification: PCR reaction system 10 μL, containing 10 ng of genomic DNA extracted in step 1, dNTP 2 mM, 10×Buffer (containing Mg 2+ ) 1.0 μL, MgCl 2 25mM, FastStart Taq enzyme 0.15U, the final concentration of each amplification primer pair is 0.1μM. The PCR primary amplification reaction conditions were: pre-denaturation at 95°C for 5 min; denaturation at 95°C for 1 min, annealing at 57°C for 1 min, extension at 72°C for 1 min, 30 cycles, and full extension at 72°C...
Embodiment 2
[0066] Example 2, in vitro detection of newborn CYP21A2 gene mutation hotspot screening
[0067] 1. Suitable for newborn heel blood test genetic screening kit (96 copies) contains the following components:
[0068] 1) PCR reaction reagent group I, including 2mM dNTP150μL, 10×PCR buffer 100μl, 25mM MgCl 2 Solution 100 μL, deionized water 200 μL, primary amplification primer mixture I 400 μL, secondary amplification primer mixture II 400 μl, 5 U / μL FastStart Taq enzyme 15 μL;
[0069] 2) Purification reagent set, including 300 μL of 1U / μL SAP enzyme, 40 μL of 5U / μL ExonI enzyme, and 260 μL of deionized water;
[0070] 3) PCR reaction reagent group II, 5×seq Buffer 120 μL, primer mixture III 100 μL, SNaPShot Mix 100 μL, deionized water 180 μL;
[0071] 4) Homozygous and heterozygous mutation positive control DNA samples are 10 μL each, and negative control sample DNA samples are 10 μL;
[0072] 5) Instruction manual.
[0073] The samples came from high-risk dried blood sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com