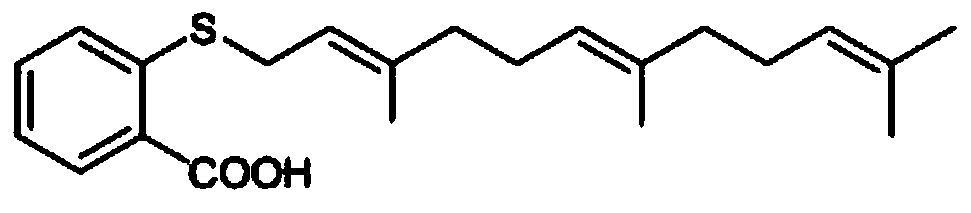
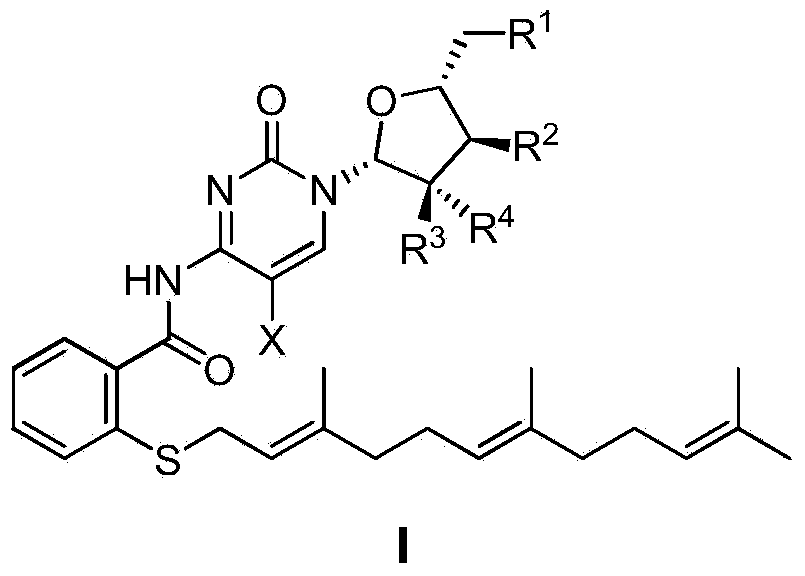
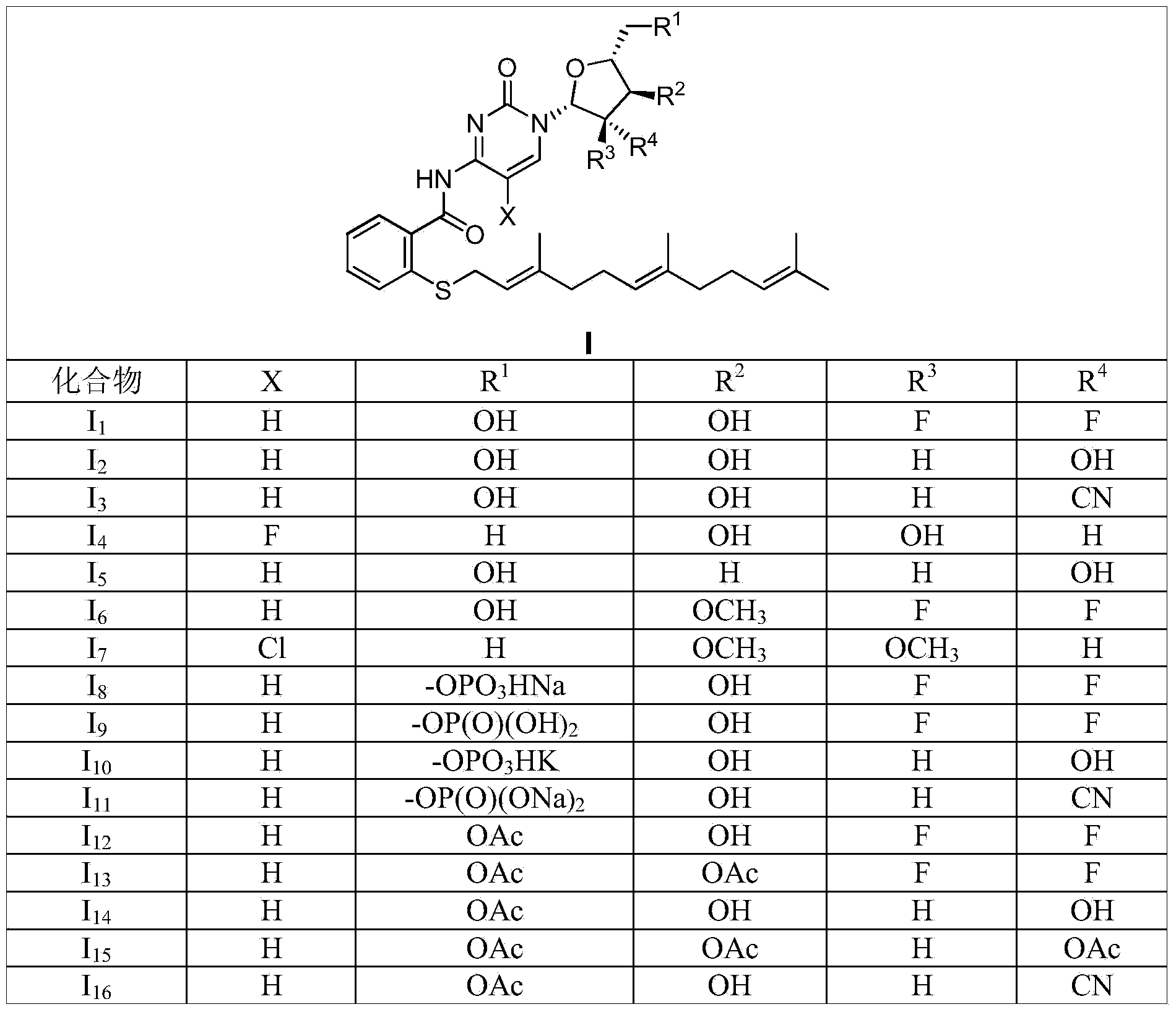
Farnesyl thiosalicylic acid-nucleoside conjugate as well as preparation method and medical application thereof
A technology of farnesylthiosalicylic acid and nucleoside conjugates, applied in the field of biomedicine, can solve the problems of increased side effects and increased burden, and achieve the effects of preventing degradation, reducing drug resistance, and promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1N-(1-((2R,4S,5R)-3,3-difluoro-4-hydroxyl-5-hydroxymethyltetrahydrofuran-2-yl)-2-one-1,2-dihydropyrimidine -4-yl)-farnesylthiosalicylic acid amide (I 1 ) preparation
[0102] 4-amino-1-((2R,4S,5R)-3,3-difluoro-4-(tert-butyldimethylsilyloxy)-5-(tert-butyldimethylsilyloxymethyl ) Tetrahydrofuran-2-yl)-1H-pyrimidin-2-one (2a) preparation
[0103] Dissolve gemcitabine (1a) (108 mg, 0.41 mmol) in 20 mL of dry DMF solution, add TBDMS-Cl (247 mg, 1.64 mmol) and 112 mg of imidazole, stir at room temperature for 10 h, evaporate the solvent under reduced pressure, and pass the crude product through silica gel column chromatography Purification (mobile phase AE:PE=1:2-2:1) gave 181mg, yield 90%.
[0104] Preparation of farnesylthiosalicylic acid chloride
[0105] Dissolve 0.54 g (1.50 mmol) of FTA in 10 mL of anhydrous CH 2 Cl 2 0.60 mL (8.27 mmol) of thionyl chloride was added thereto, stirred at 55°C for 1 h, and concentrated to obtain farnesylthiosalicylic acid ch...
Embodiment 2
[0110] Example 2N-(1-((2R,3R,4R,5R)-3,4-dihydroxy-5-hydroxymethyltetrahydrofuran-2-yl)-2-one-1,2-dihydropyrimidine-4 -yl)-farnesylthiosalicylic acid amide (I 2 ) preparation
[0111] 4-Amino-1-((2R,3R,4R,5R)-3,4-bis(tert-butyldimethylsilyloxy)-5-(tert-butyldimethylsilyloxymethyl)tetrahydrofuran Preparation of -2-yl)-1H-pyrimidin-2-one (2b)
[0112] Referring to the preparation method of compound (2a) in Example 1, the pale yellow oily substance (2b) was prepared by reacting TBDMS-Cl with cytarabine (1b) instead of gemcitabine in the method, with a yield of 72%.
[0113] N-(1-((2R,3R,4R,5R)-3,4-bis(tert-butyldimethylsilyloxy)-5-(tert-butyldimethylsilyloxymethyl)tetrahydrofuran- Preparation of 2-yl)-2-keto-1,2-dihydropyrimidin-4-yl)-farnesylthiosalicylic acid amide (3b)
[0114] With reference to the preparation method of compound (3a) in Example 1, the compound (2a) in the alternative method of compound (2b) reacts with farnesyl thiosalicylic acid chloride to obtain light y...
Embodiment 3
[0117] Example 3 N-(1-((2R, 3R, 4R, 5R)-3-cyano-4-hydroxyl-5-hydroxymethyltetrahydrofuran-2-yl)-2-one-1,2-dihydropyrimidine -4-yl)-farnesylthiosalicylic acid amide (I 3 ) preparation
[0118] 4-amino-1-((2R,3R,4R,5R)-3-cyano-4-(tert-butyloxycarbonyloxy)-5-(tert-butyloxycarbonyloxymethyl)tetrahydrofuran-2 Preparation of -yl)-1H-pyrimidin-2-one (2c)
[0119] 4-amino-1-((2R,3R,4R,5R)-3-cyano-4-hydroxyl-5-hydroxymethyltetrahydrofuran-2-yl)-1H-pyrimidin-2-one (1c) (103mg, 0.41mmol) was dissolved in 10mL of 1,4-dioxane and 15ml of 1N potassium hydroxide aqueous solution, and Boc anhydride (1.48g, 6.8mmol) was slowly added dropwise in 10mL of 1,4-dioxane solution, return to room temperature and stir for 10 h after dropping, evaporate the solvent under reduced pressure after spot plate reaction, add 25ml of water, extract the water layer with 20ml of ethyl acetate three times, combine the organic layers and wash with saturated saline, dry and concentrate under reduced pressure The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com