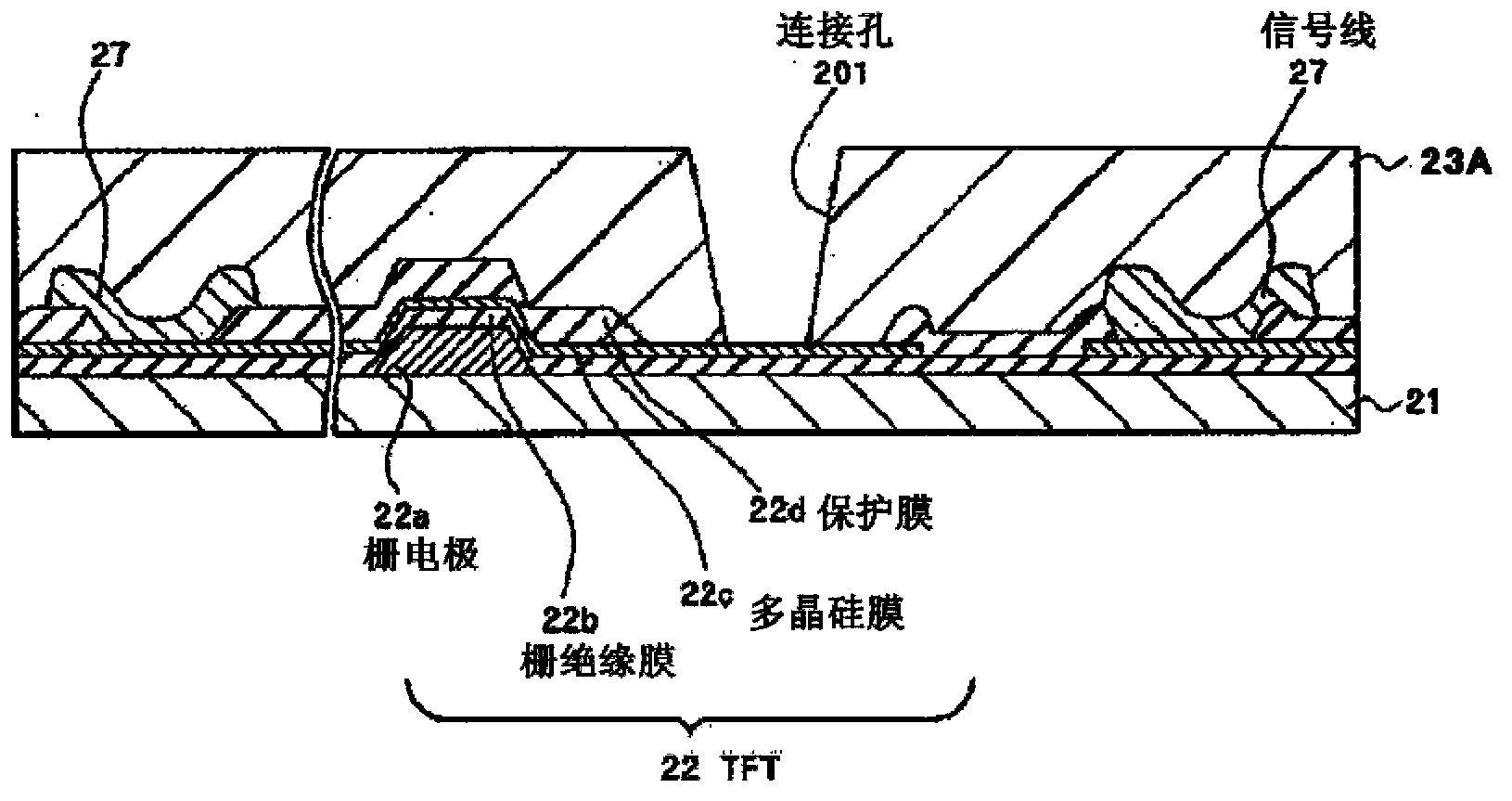
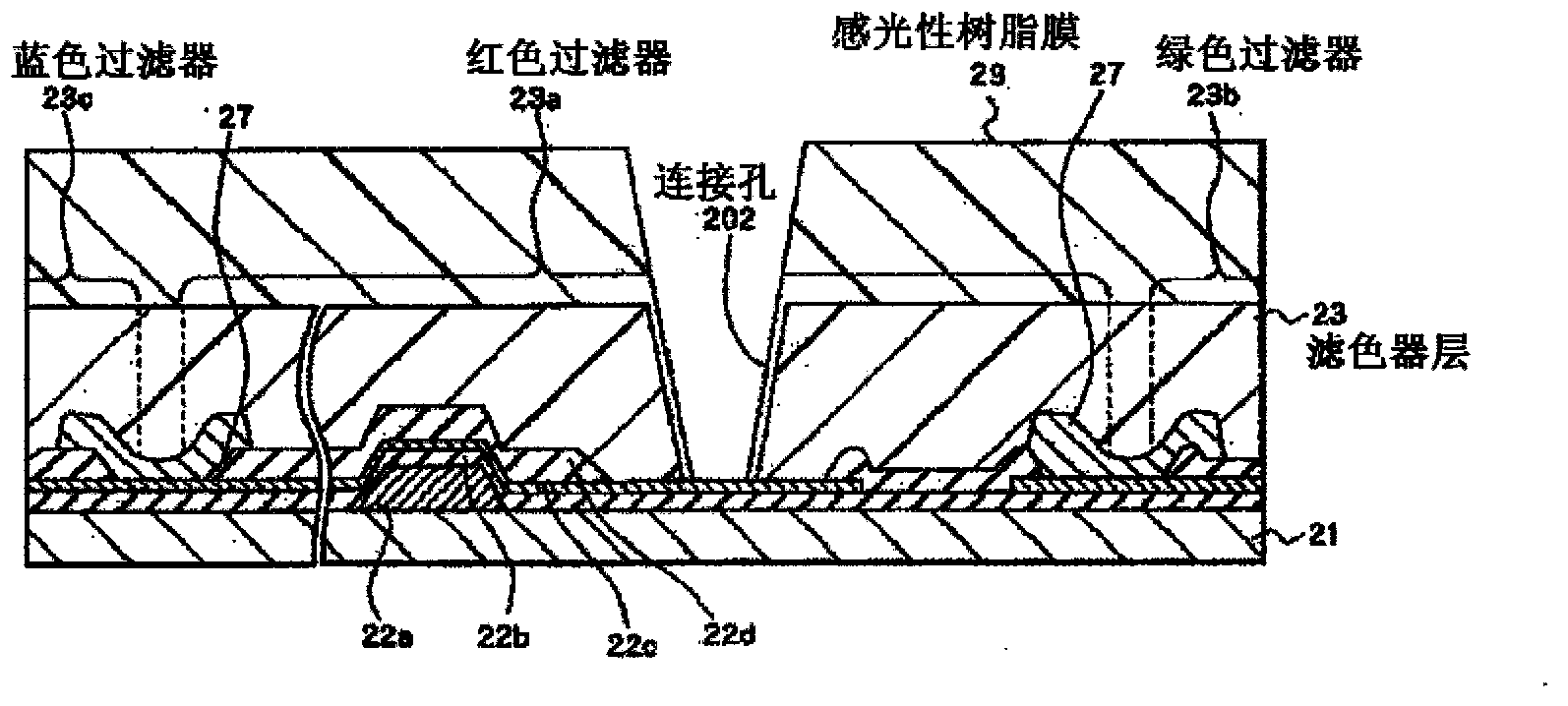
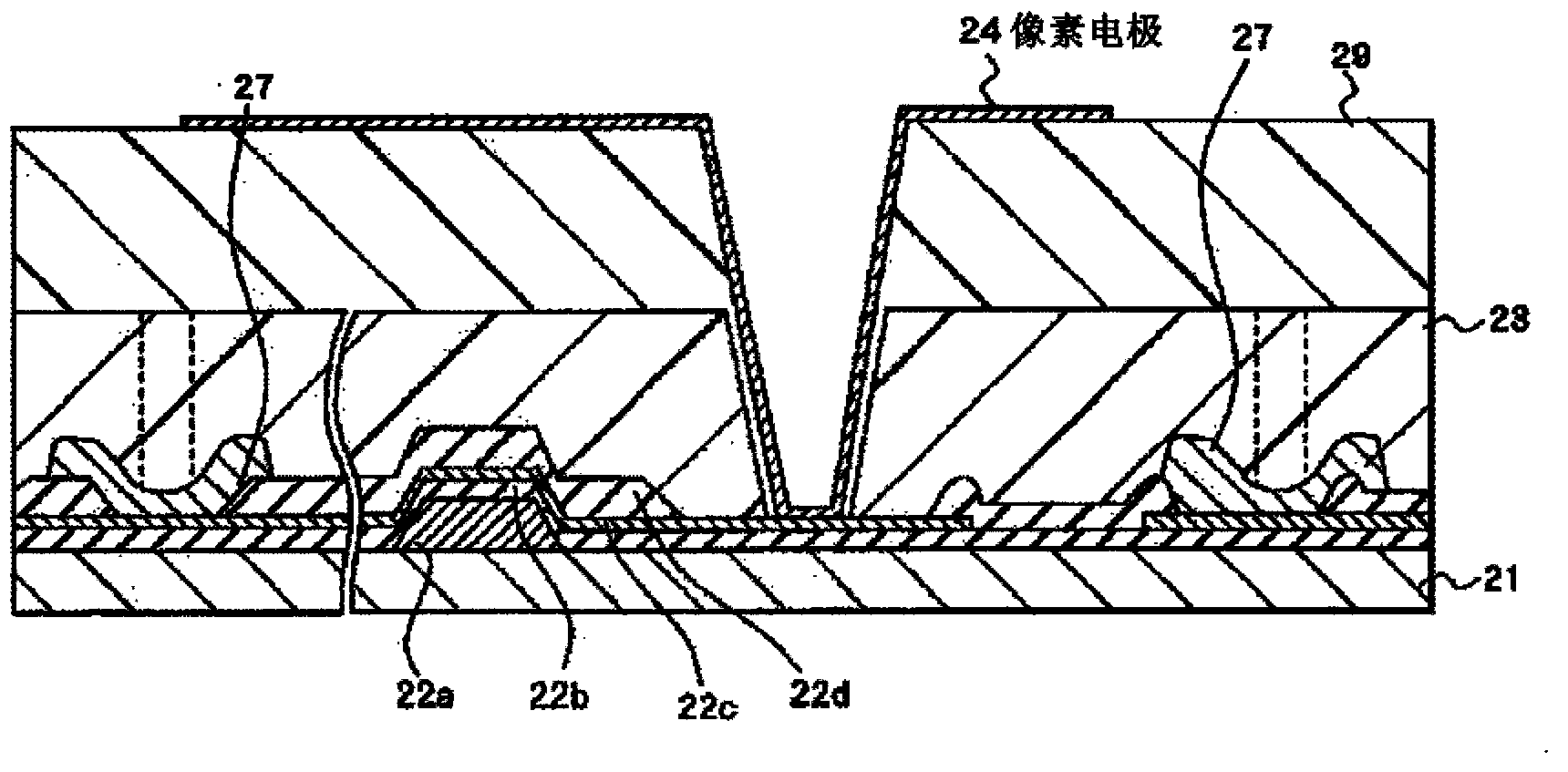
Colored curable resin composition
A technology of curable resin and composition, which is applied in nonlinear optics, instruments, exposure devices for photo-plate making process, etc., and can solve the problems that the contrast of color filters may not be able to fully meet the needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0396] Hereinafter, the colored curable resin composition of the present invention will be described in more detail through examples.
[0397] Unless otherwise specified, "%" and "part" in an example are mass % and a mass part.
[0398] In the following synthesis examples, compounds were identified by mass spectrometry (LC; Agilent 1200 type, MASS; Agilent LC / MSD type) or elemental analysis (VARIO-EL; (Elementar Co., Ltd.)).
Synthetic example 1
[0400] 15 parts, To 150 parts of chloroform and 8.9 parts of N,N-dimethylformamide, 10.9 parts of thionyl chloride were added dropwise while maintaining the temperature below 20°C under stirring. After completion of the dropwise addition, the temperature was raised to 50°C, and the same temperature was maintained for 5 hours to allow a reaction, and then cooled to 20°C. While maintaining the cooled reaction solution at 20° C. or lower with stirring, a mixed solution of 12.5 parts of 2-ethylhexylamine and 22.1 parts of triethylamine was added dropwise. Then, it stirred at the same temperature for 5 hours and made it react. Next, after distilling off the solvent from the obtained reaction mixture with a rotary evaporator, a small amount of methanol was added and vigorously stirred. This mixture was added to a mixed liquid of 375 parts of ion-exchanged water while stirring, and crystals were precipitated. The precipitated crystals were filtered, thoroughly washed with ion-exch...
Synthetic example 2
[0403] 20 parts of the compound represented by the formula (1x) and 200 parts of N-ethyl-o-toluidine (manufactured by Wako Pure Chemical Industries, Ltd.) were mixed under light-shielding conditions, and the resulting solution was stirred at 110° C. for 6 hours. After cooling the obtained reaction liquid to room temperature, it was added to the liquid mixture of 800 parts of water and 50 parts of 35% hydrochloric acid, and it stirred at room temperature for 1 hour, and a crystal precipitated. The precipitated crystal was obtained as a suction-filtered residue and dried to obtain 24 parts of the compound represented by the formula (1-24). The yield is 80%.
[0404]
[0405] The structure of the compound represented by the formula (1-24) was confirmed by mass spectrometry (LC; Agilent 1200 type, MASS; Agilent LC / MSD type).
[0406] (mass analysis) ionization mode = ESI+: m / z = [M+H] + 603.4
[0407] Exact Mass: 602.2
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com