Death ligand antibody conjugated polyethylene glycol modified lipid nano drug delivery system and its application
A polyethylene glycol and death ligand technology, applied in the field of biomedicine, can solve problems such as the targeting of ischemic lesions that are rarely considered, and achieve the goal of improving the efficiency of brain entry, good biocompatibility, and increasing the amount of distribution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment one Establishment of brain-targeted nano drug delivery system coupled with Fas ligand antibody
[0030] 1.1 Synthesis of rhodamine stearylamine
[0031] Dissolve 25.14g of stearylamine and 50mg of Rhodamine B in 6mL of ethanol, and react in the dark for 24 hours under magnetic stirring at 400rpm, then add 50mL of water, and freeze-dry. The freeze-dried sample was washed with 5 mL of distilled water, and then filtered through a 0.45 μm filter membrane. Dry at room temperature to obtain rhodamine stearylamine.
[0032] 1.2 Preparation of drug-loaded lipid nanoparticles
[0033] Dissolve 13.5 mg of glyceryl monostearate, 8.5 mg of medium-chain triglycerides, 1.5 mg of rhodamine stearylamine, and 5 mg of butylphthalide in 1 ml of ethanol, heat and stir in a water bath at 70°C to dissolve. Slowly inject the above solution into 9 ml of 0.1% Tween 80 aqueous solution while hot, and stir magnetically at 400 rpm for 5 minutes to obtain a 3 mg / ml lipid nanoparticl...
Embodiment 2
[0038] Embodiment two Evaluation of physicochemical properties of brain-targeted lipid nanoparticles coupled with Fas ligand antibody
[0039] 2.1 Determination of particle size and potential of lipid nanoparticles
[0040] Take 0.5 ml of the unmodified, Fas ligand antibody-coupled and PEG-modified two drug-loaded lipid nanoparticles prepared above, dilute 10 times with deionized water, and use a particle size and surface potential analyzer (Zetasizer, 3000HS, Malvern Instruments ) to measure the particle size and potential of the two lipid nanoparticles, respectively.
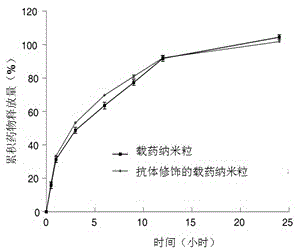
[0041] The results showed that the diameter of antibody-modified drug-loaded nanoparticles (60.97±7.95nm) was larger than that of non-antibody-modified drug-loaded nanoparticles (38.23±3.22nm), and the space diameter of nanoparticles after antibody modification increased; resemblance.
[0042] 2.2 Drug encapsulation efficiency and drug loading
[0043] The content of butylphthalide in lipid nanoparticl...
Embodiment 3
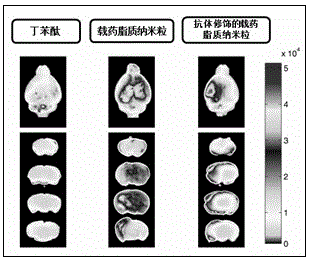
[0052] Embodiment Three Pharmacodynamics of brain-targeted drug-loaded nanoparticles against ischemic brain injury
[0053] 3.1 The pathological model of ischemic brain injury was established by the method of middle cerebral artery suture embolization in mice
[0054] The model of cerebral microvascular ischemia injury was established by using the focal middle cerebral artery occlusion / reperfusion (MCAO) model in mice: the mice were anesthetized with 3% isoflurane, fixed in supine position, and the neck was disinfected , Carotid median incision, separation of the left common carotid artery, internal carotid, external carotid artery. The proximal end of the common carotid artery and the external carotid artery were ligated, the internal carotid artery was clamped with an arterial clamp, a small opening was cut in the common carotid artery, and a nylon thread plug (smooth spherical surface, thread plug diameter 0.26 mm) was inserted horizontally toward the end of the internal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com