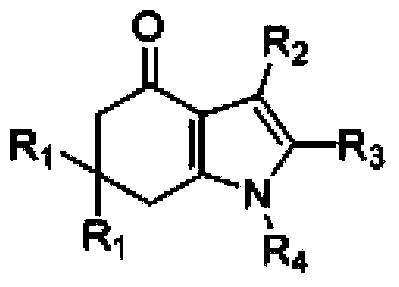
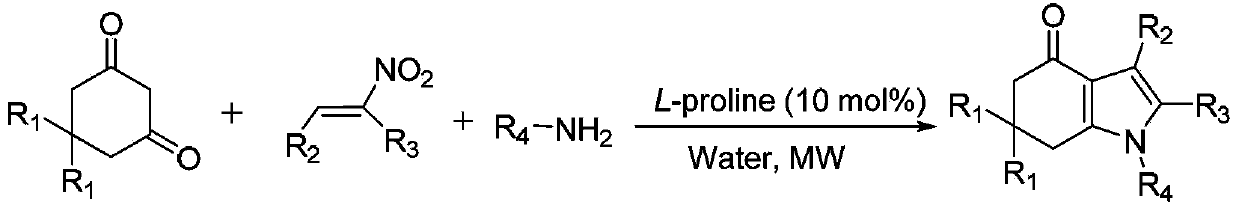Tetrahydroindole compound, and preparation method and application thereof
A tetrahydroindoline compound and compound technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of relatively little functionalization research, poor regioselectivity, and easy side reactions, etc., and achieve good practicability And the effects of economic value, yield and purity improvement, high practical value and academic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
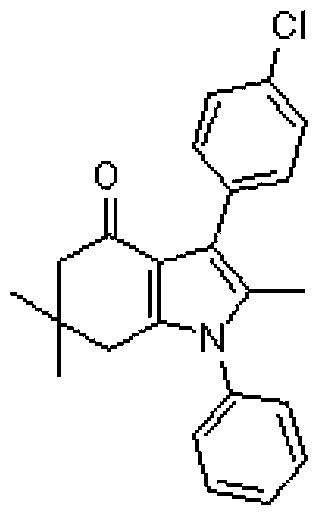
[0036] Weigh 0.5mmol of 5,5-dimethyl-1,3-cyclohexanedione, 0.5mmol of aniline, 0.5mmol of (E)-β-nitro-β-methyl-p-chlorostyrene and 0.05mmol of Add L-proline into the reactor, and then add 3mL of water, and act under microwave for 10 minutes. After the reaction was completed, it was cooled to room temperature, extracted with ethyl acetate, and separated by column chromatography with petroleum ether / ethyl acetate to obtain an analytically pure product with a yield of 83%.
[0037] Product Confirmation:
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 ):δ=7.58-7.50(m,4H,ArH),7.40-7.33(m,5H,ArH),2.45(s,2H,CH 2 ),2.44(s,2H,CH 2 ),2.38(s,3H,CH 3 ),1.08(s,6H,CH 3 ). 13 C NMR (100MHz, CDCl 3 ):δ=194.5,146.2,143.2,137.1,132.0,131.7,129.6,129.0,128.8,128.7,128.2,127.8,127.7,53.1,37.0,35.2,28.5,11.1.HRMS(ESI)C 23 h 23 ClNO:[M+H] + calcd 364.1468, found: 364.1463.
Embodiment 2
[0041] Weigh 0.5mmol of 5,5-dimethyl-1,3-cyclohexanedione, 0.5mmol of p-chloroaniline, 0.5mmol of (E)-β-nitro-β-methyl-p-methylstyrene and 0.05 mmol of L-proline were added to the reactor, and then 3 mL of water was added, and the reaction was performed under microwave for 15 minutes. After the reaction was completed, it was cooled to room temperature, extracted with ethyl acetate, and separated by column chromatography with petroleum ether / ethyl acetate to obtain an analytically pure product with a yield of 79%.
[0042] Product Confirmation:
[0043]
[0044] 1 H NMR (400MHz, CDCl 3):δ=7.54-7.52(m,2H,ArH),7.32(d,J=8.0Hz,2H,ArH),7.24(d,J=8.8Hz,2H,ArH),7.19(d,J=7.6 Hz,2H,ArH),2.43(s,2H,CH 2 ),2.38(s,3H,CH 3 ),2.37(s,2H,CH 2 ),2.01(s,3H,CH 3 ),1.09(s,6H,CH 3 ). 13 C NMR (100MHz, CDCl 3 ):δ=193.0,142.6,135.9,135.8,134.7,131.3,130.2,129.8,129.1,128.4,128.2,120.6,116.7,53.1,37.1,35.2,28.5,21.3,11.1.HRMS(ESI)C 24 h 25 ClNO:[M+H] + calcd 378.1625, found: 378.1619.
Embodiment 3
[0046] Weigh 0.5mmol of 5,5-dimethyl-1,3-cyclohexanedione, 0.5mmol of cyclopropylamine, 0.5mmol of (E)-β-nitro-β-methylstyrene and 0.05mmol of L-proline was added to the reactor, and then 3 mL of water was added, and the reaction was performed under microwave for 8 minutes. After the reaction was completed, it was cooled to room temperature, extracted with ethyl acetate, and separated by column chromatography with petroleum ether / ethyl acetate to obtain an analytically pure product with a yield of 85%.
[0047] Product Confirmation:
[0048]
[0049] 1 H NMR (400MHz, CDCl 3 ):δ=7.37-7.34(m,4H,ArH),7.26-7.24(m,1H,ArH),3.03-3.00(m,1H,CH),2.80(s,2H,CH 2 ),2.32(s,2H,CH 2 ),2.28(s,3H,CH 3 ),1.91(s,3H,CH 3 ),1.17-1.13(m,8H,CH 3 ,CH 2 ),1.00-0.96(m,2H,CH 2 ). 13 C NMR (100MHz, CDCl 3 ):δ=193.0,144.1,134.8,130.4,129.9,127.5,126.1,120.0,115.9,53.0,37.6,35.1,28.7,26.0,11.3,7.6. HRMS(ESI)C 20 h 24 NO:[M+H] + calcd 294.1858, found: 294.1852.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com