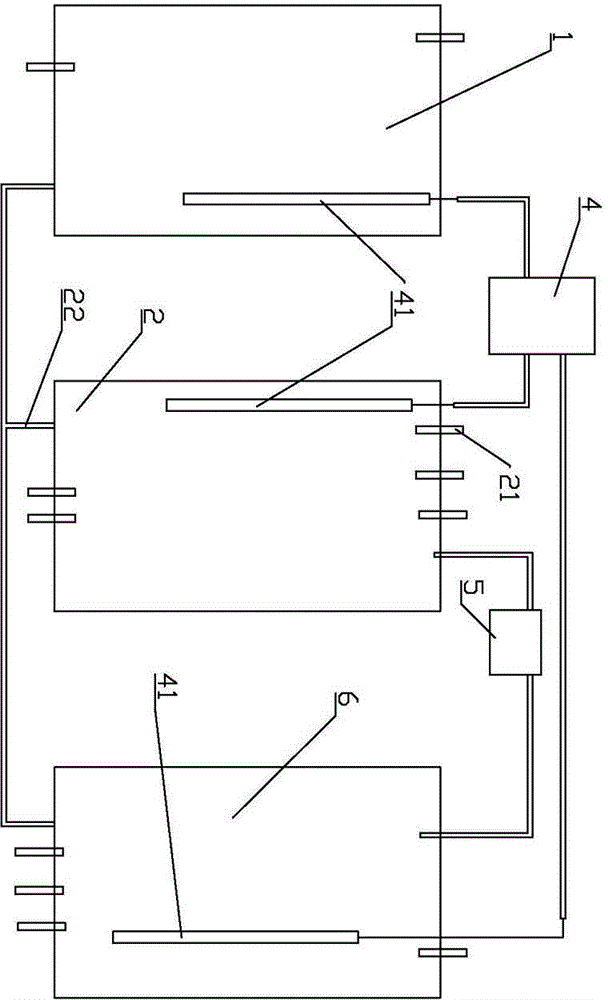
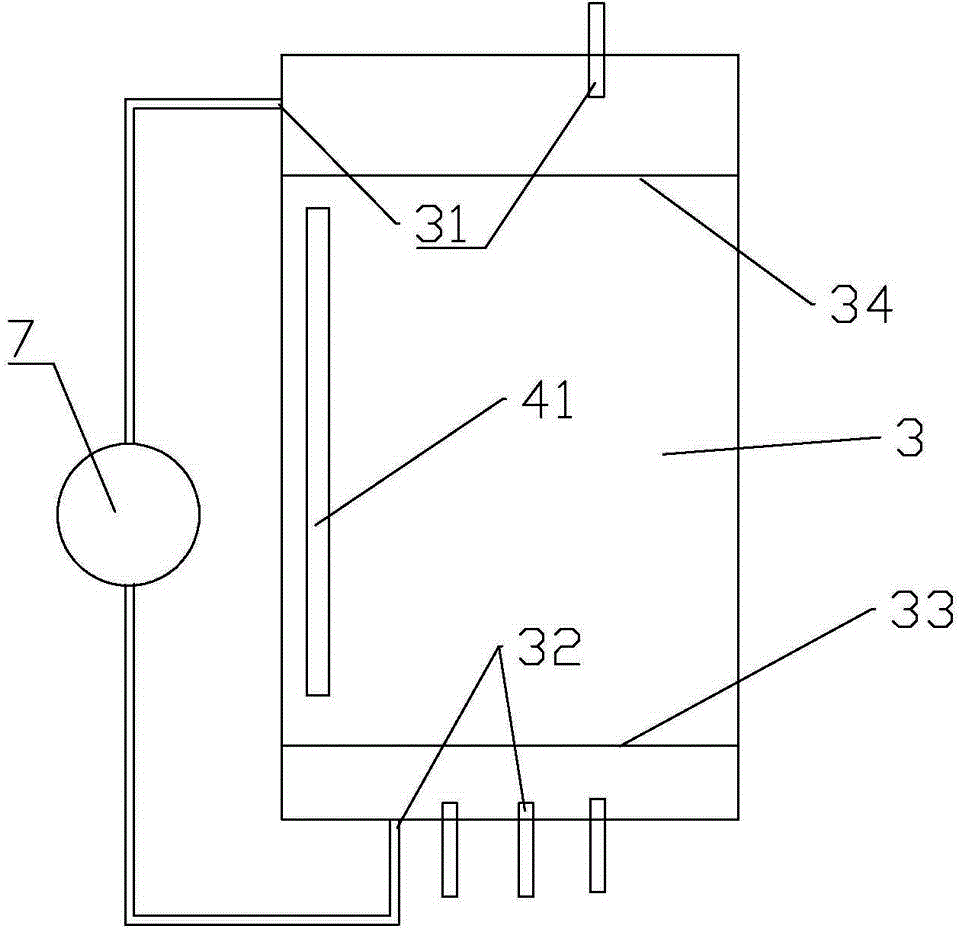
Method and system for preparing high-purity lithium carbonate
A preparation system, lithium carbonate technology, applied in lithium carbonate;/acid carbonate, general water supply saving, energy input and other directions, can solve the problems of energy, fresh water shortage, low efficiency, large influence of natural conditions, etc. To achieve the effect of reducing ecological problems, easy to use, and high energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of high-purity lithium carbonate comprises the steps:
[0041] 1) Concentrate the carbonated salt lake brine, then raise the temperature to precipitate lithium carbonate saturated crystals, collect the crystals to obtain lithium carbonate refined salt;
[0042] 2) Rinse the collected lithium carbonate fine salt with the distilled water recovered when the brine is evaporated and concentrated above 60°C, and dissolve the sodium potassium salt therein;
[0043] 3) drying to obtain high-purity lithium carbonate.
[0044] Preferably, the salt lake brine is concentrated by evaporation under reduced pressure, and then the temperature is raised to above 60°C, preferably above 65°C or 70°C to precipitate lithium carbonate crystals. The higher the crystallization temperature, the more conducive to the precipitation of higher-purity lithium carbonate refined salt, which is conducive to reducing the difficulty of subsequent purification operations.
[0045]...
Embodiment 1
[0058] Li ion concentration 1.29g / L brine, above 60°C crystallization precipitates coarse lithium salt and brine mixed wet salt, simply removes the supernatant and weighs 897.6g, mixes with 80°C distilled water 3000g at one time and fully stirs for 3 minutes, after Suction filtration and oven dry, obtain the lithium carbonate 47.4g that purity is 62%, obtain lithium carbonate yield from thick lithium salt and be 73.5%.
Embodiment 2
[0060] Li ion concentration 1.29g / L brine, above 60°C crystallizes and precipitates coarse lithium salt and brine mixed wet salt, simply removes the supernatant and weighs 877.9g, uses 70°C distilled water 2250g, respectively 1200g, 600g, 300g, 150g A total of 4 times were rinsed in the manner of Example 1, and each fully stirred for 30 seconds for a total of 2 minutes. After suction filtration and drying, the dry purity obtained was 91.3% Lithium Retard 37.56g, and Lithium Retard yield was obtained from the thick lithium salt. was 85.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com