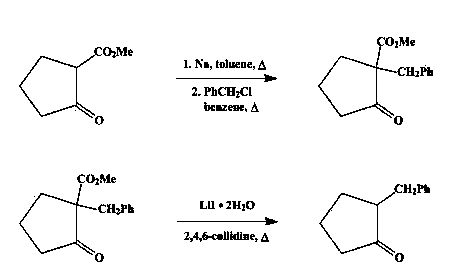
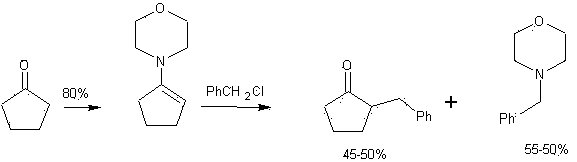
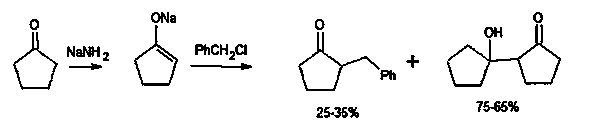
Synthetic method for 2-benzyl cyclopentanone
A technology of benzylcyclopentanone and a synthesis method is applied in the field of preparation of organic pharmaceutical intermediates, and can solve the problems of sodium amide easily causing fire, low total product yield, useless by-products, etc. cost, waste reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Friedel-Crafts acylation and esterification (synthesis of methyl 5-benzoylvalerate, ROH=CH30H):
[0046] Add 80ml of benzene and 0.22mol of aluminum trichloride into the reaction flask, add dropwise 0.1mol of adipic anhydride (dissolved in 20ml of benzene) at 31-55°C, then react at 40-55°C for 2 hours, add dropwise 0.5mol of methanol, Continue to react at 40-55°C for 2 hours, add the reaction mixture to 200ml of water for hydrolysis, statically separate layers, wash the benzene layer with aqueous sodium carbonate solution, distill benzene at 100°C, and the residue is 5-benzoylvaleric acid methyl Ester, the product is light yellow, the freezing point is 35-36.5°C, the content is over 98%, and the yield is 90-92%.
[0047] 2) Condensation and cyclization reaction (synthesis of 2-benzoylcyclopentanone, tripotassium phosphate method) 0.1mol methyl 5-benzoylvalerate, 0.1mol tripotassium phosphate and 50ml DMF were refluxed for 12 hours, Cool down to about 30°C, remove...
Embodiment 2
[0051] 1) Friedel-Crafts acylation and esterification (synthesis of methyl 5-benzoylvalerate, ROH=CH3OH):
[0052] Add 80ml of benzene and 0.22mol of aluminum trichloride into the reaction flask, add dropwise 0.1mol of adipic anhydride (dissolved in 20ml of benzene) at 31-55°C, then react at 50-70°C for 2 hours, add dropwise of 0.5mol of methanol, Continue to react at 50-70°C for 2 hours, add the reaction mixture to 200ml of water for hydrolysis, statically separate layers, wash the benzene layer with aqueous sodium carbonate solution, distill benzene at 100°C, and the residue is 5-benzoylvaleric acid methyl Ester, the product is light yellow, the freezing point is 35-36.5°C, the content is over 98%, and the yield is 90-92%.
[0053] 2) Condensation cyclization reaction (synthesis of 2-benzoylcyclopentanone, triethylamine method)
[0054]0.1mol of methyl 5-benzoylvalerate, 0.1mol of triethylamine and 50ml of DMF were put into the autoclave,
[0055] React at 150-155°C for ...
Embodiment 3
[0059] 1) Friedel-Crafts acylation and esterification (synthesis of methyl 5-benzoylvalerate):
[0060] Add 80ml of benzene and 0.3mol of aluminum trichloride into the reaction flask, add dropwise 0.1mol of adipic anhydride (dissolved in 20ml of benzene) at 31-55°C, then react at 60-75°C for 2 hours, add dropwise of 0.4mol of ethanol, Continue to react at 60-75°C for 2 hours, add the reaction mixture to 200ml of water for hydrolysis, statically separate layers, wash the benzene layer with aqueous sodium carbonate solution, distill benzene at 100°C, and the residue is 5-benzoylvaleric acid methyl Ester, the product is light yellow, the freezing point is 35-36.5°C, the content is over 98%, and the yield is 90-92%.
[0061] 2) Condensation and cyclization reaction (synthesis of 2-benzoylcyclopentanone, tripotassium phosphate method) 0.2mol of methyl 5-benzoylvalerate, 0.2mol of tripotassium phosphate and 1000ml of DMF were refluxed for 12 hours, Cool down to about 30°C, remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Freezing point | aaaaa | aaaaa |
| Freezing point | aaaaa | aaaaa |
| Freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com