Quinine drug-vincristine drug co-carried liposome and preparation method thereof
A technology of vincristine and vincristine sulfate, applied in liposome delivery, drug combination, pharmaceutical formulation, etc., can solve the problem of interaction reduction of multi-drug resistance of anti-tumor drugs, anti-cancer effect of cell drug resistance, Can not be administered orally, etc., to overcome multidrug resistance, wide selection of phospholipids, and improve lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
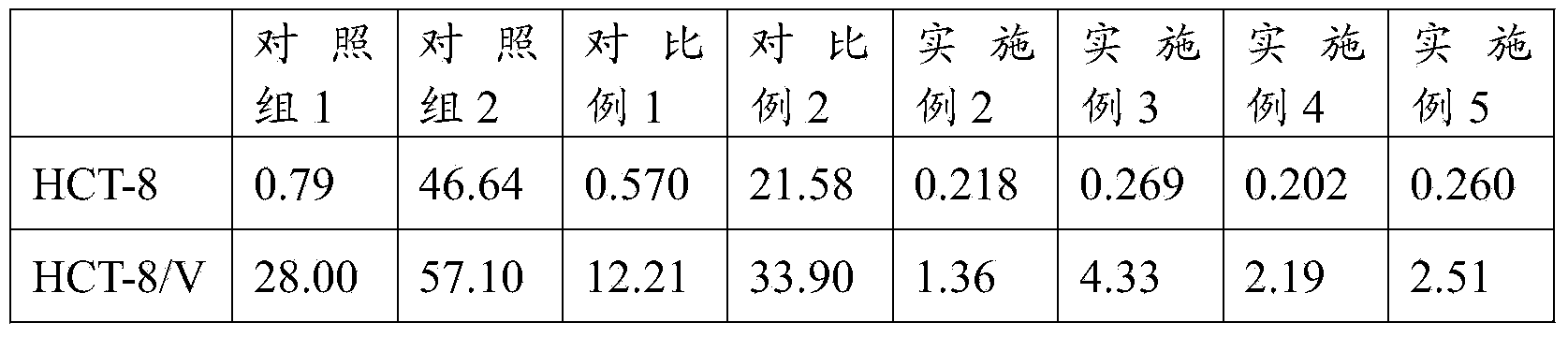
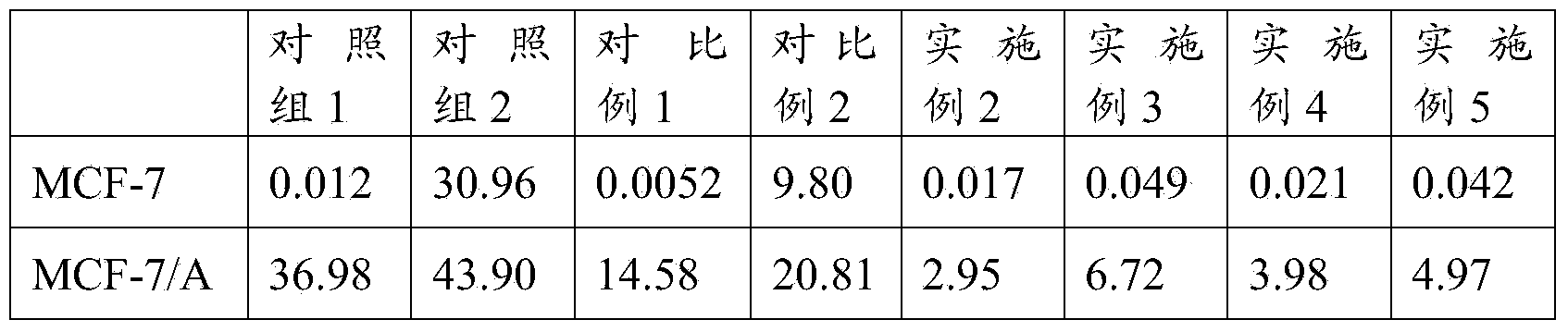
[0037] Weigh 350mg of soybean lecithin (phosphatidylcholine purity>76%) and 70mg of cholesterol dissolved in 6mL of absolute ethanol, then add 20mg of vincristine and 20mg of quinine hydrochloride (namely vincristine) dissolved in 4ml of absolute ethanol and quinine hydrochloride were dissolved in absolute ethanol, the same below), fully dissolved and mixed evenly, then the organic solvent ethanol was evaporated under reduced pressure, so that phospholipids and other film-forming materials formed a uniform lipid film at the bottom of the flask, and then 5mL PBS was added (Phosphate, pH=7.4) buffer solution, vortexed for 5 minutes, and finally sonicated for 10 minutes with a cell disruptor to obtain 5ml of vincristine and quinine co-loaded liposome suspension, and the co-loaded liposome suspension of vincristine and quinine was measured. The average particle diameter (number average) of the liposome suspension is 38.6nm. The liposome and unencapsulated free drug are separated by...
Embodiment 2
[0039] Weigh 400mg of soybean lecithin (phosphatidylcholine purity>76%) and 100mg of cholesterol dissolved in 6mL of absolute ethanol, then add 20mg of vincristine and 40mg of quinine hydrochloride dissolved in 4ml of absolute ethanol, fully dissolve and mix Evenly, then evaporate the organic solvent ethanol under reduced pressure, so that the film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask, then add 5mL PBS (phosphate, pH=7.4) buffer solution, vortex for 5min, and finally use a cell disruptor to sonicate 10min, obtain 5ml vincristine and quinine co-carried liposome suspension, record the average particle diameter (number average) of this vincristine and quinine co-carried liposome suspension to be 45.8nm, separate by dialysis Liposome and unencapsulated free drug, vincristine encapsulation rate was 83.90%, drug loading was 3.36%.
Embodiment 3
[0041] Weigh 400mg of soybean lecithin (phosphatidylcholine purity>76%) and 80mg of cholesterol dissolved in 6mL of absolute ethanol, then add 20mg of vincristine and 10mg of quinine hydrochloride dissolved in 4ml of absolute ethanol, fully dissolve and mix Evenly, then evaporate the organic solvent ethanol under reduced pressure, so that the film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask, then add 5mL PBS (phosphate, pH=7.4) buffer solution, vortex for 5min, and finally use a cell disruptor to sonicate 10min, obtain 5ml vincristine and quinine co-carried liposome suspension, record the average particle diameter (number average) of this vincristine and quinine co-carried liposome suspension to be 35.7nm, separate by dialysis Liposomes and unencapsulated free drugs had an encapsulation rate of vincristine of 79.20% and a drug loading capacity of 3.30%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com