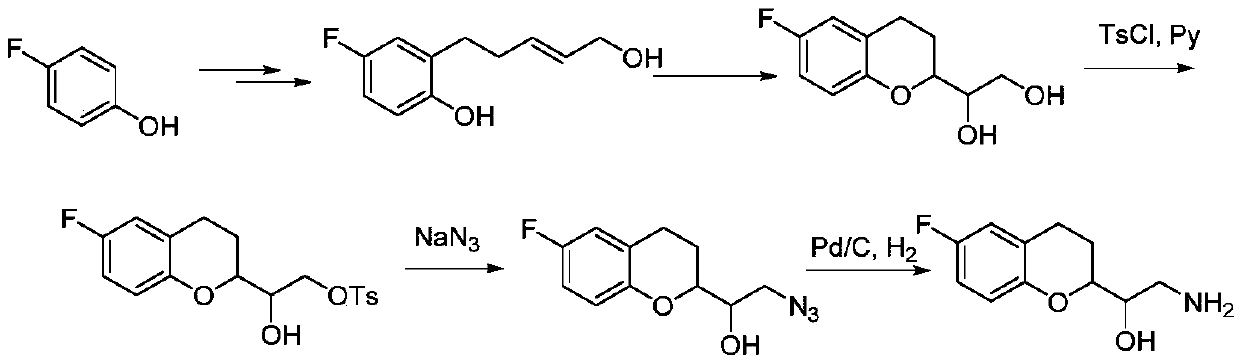
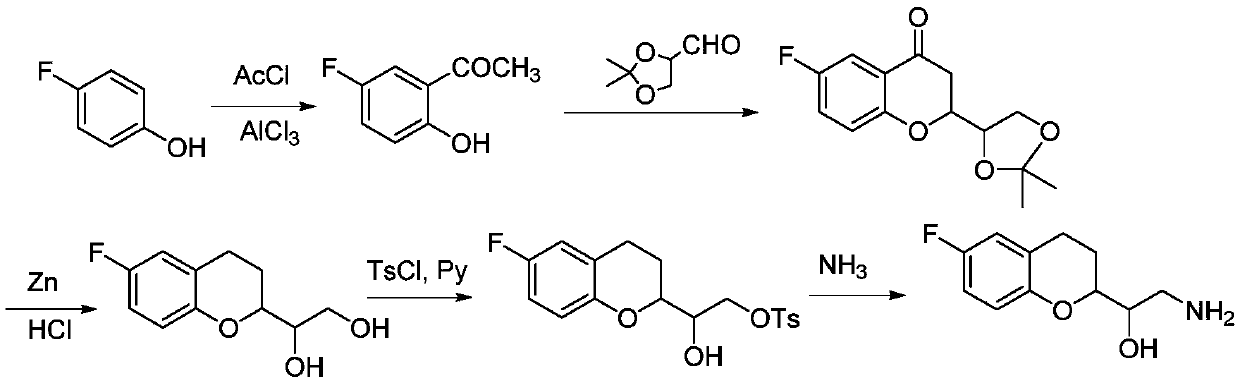
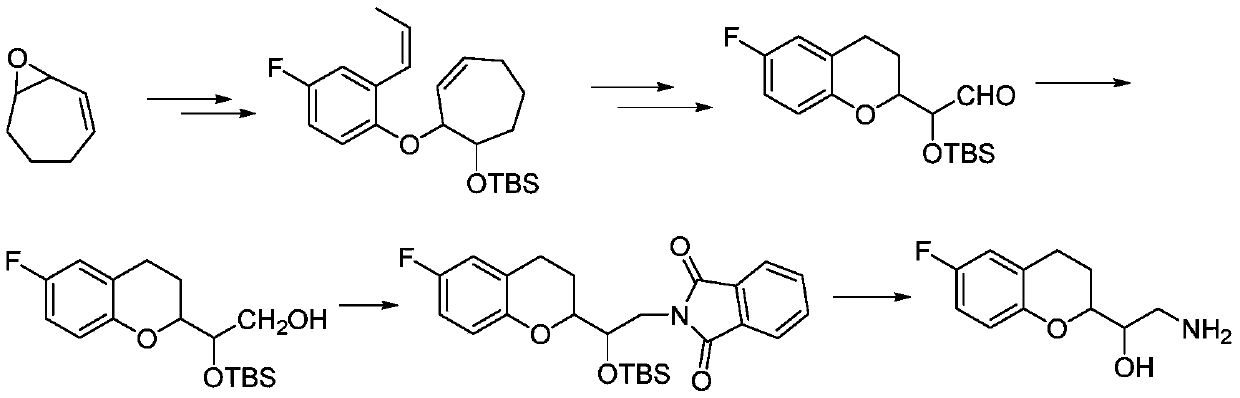
Synthetic method of 2-amino-1-(-6-fluoro-2-chromanyl)ethanol
A synthetic method, the technology of chroman, applied in the field of preparation of 2-amino-1-ethanol compounds, can solve the problems of many reaction steps, harsh reaction conditions, low yield, etc., achieve mild conditions, easy operation, and synthetic route short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 6-Fluorochroman-2-carboxylic acid (1 g) was dissolved in THF (10 mL), carbonyldiimidazole (1 g) was added with stirring at room temperature, and the mixture was heated to reflux for 1 to 5 hours to obtain an acyl imidazole solution. Potassium tert-butoxide (1 g) and THF (15 mL) were mixed, and nitromethane (2 mL) was added dropwise with stirring at room temperature, and stirred at room temperature for 1 to 5 hours. Mix the acyl imidazole solution and nitromethane solution, heat and reflux for 3 to 20 hours, cool, add water (50mL), adjust the pH value to acidic with hydrochloric acid, add ethyl acetate (50mL) for extraction, wash the organic phase with water, dry, spin The solvent was distilled off to obtain a crude product, which was recrystallized from ethanol to obtain a white solid (III) (0.92 g, yield 75%), melting point 69-71 °C,1 HNMR (400MHz, CDCl 3 )δ2.08-2.13(m,1H),2.33-2.35(m,1H),2.80-2.89(m,2H),4.69(dd,J=9.5,3.2Hz,1H),5.52(d,J= 16.0Hz, 1H), 5.68(d, J=16.0Hz,...
Embodiment 2
[0053] Mix 6-fluorochroman-2-carboxylic acid (1g) with toluene (10mL), add thionyl chloride (1mL), install a gas absorption device, and heat to reflux for 1 to 5 hours. Remove the solvent and unreacted thionyl chloride under reduced pressure, add dichloromethane (10 mL), add imidazole (0.7 g), and stir at room temperature for 1 to 5 hours. Potassium tert-butoxide (1 g) and THF (15 mL) were mixed, and nitromethane (2 mL) was added dropwise with stirring at room temperature, and stirred at room temperature for 1 to 5 hours. Mix the acyl imidazole solution and nitromethane solution, heat and reflux for 3 to 20 hours, cool, add water (50mL), adjust the pH value to acidic with hydrochloric acid, add ethyl acetate (50mL) for extraction, wash the organic phase with water, dry, spin The solvent was distilled off to obtain a crude product, which was recrystallized from ethanol to obtain a white solid (III) (0.98g, yield 80%), melting point 69-71°C, 1 HNMR (400MHz, CDCl 3 )δ2.08-2.13(...
Embodiment 3
[0055] Dissolve the product (III) (0.92g) obtained in Example 1 in THF (20mL), drop it into a mixture of lithium aluminum hydride (0.4g) and THF (20mL) cooled in an ice-water bath, and continue stirring for 1~ 5 hours. Slowly add water to terminate the reaction, extract three times with dichloromethane, dry the extract, and rotary evaporate to obtain the product 2-amino-1-(-6-fluoro-2-chromanyl)ethanol (IV) (0.51g, yield 63 %). 1 HNMR (400MHz, CDCl 3 )δ1.87-1.99 (m, 4H), 2.73-3.00 (m, 4H), 3.63-3.67 (m, 1H), 3.94-3.98 (m, 1H), 6.73-6.79 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com