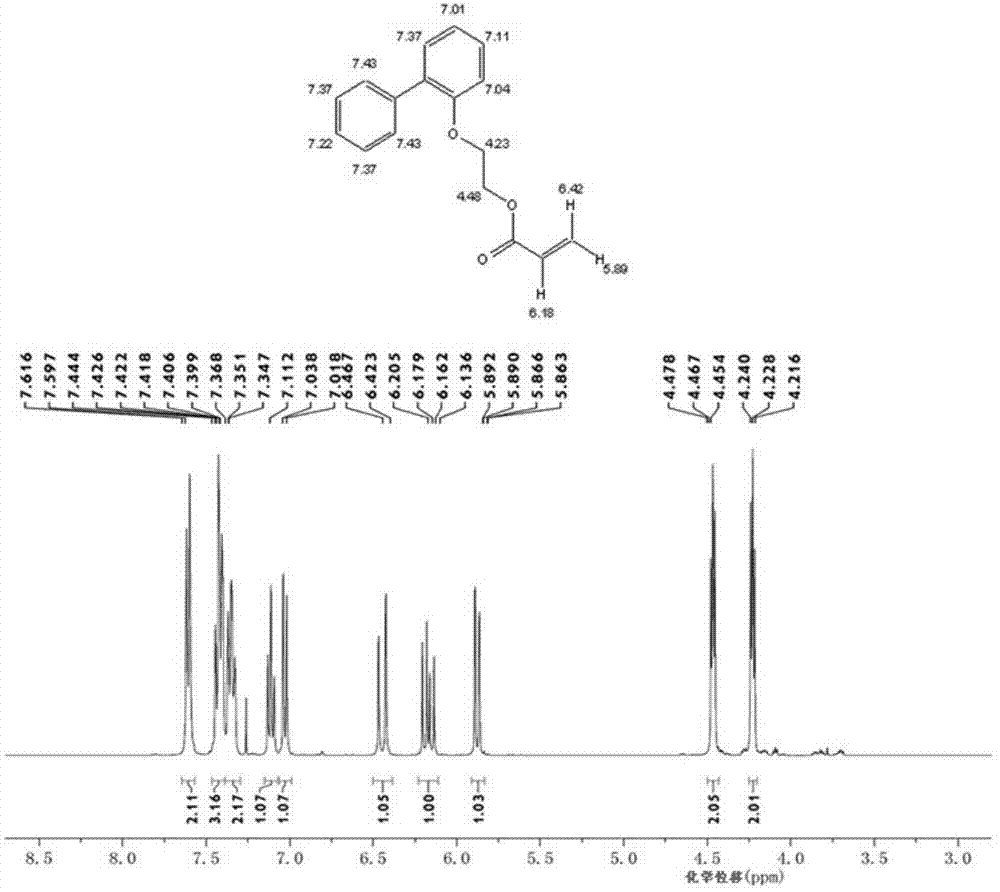
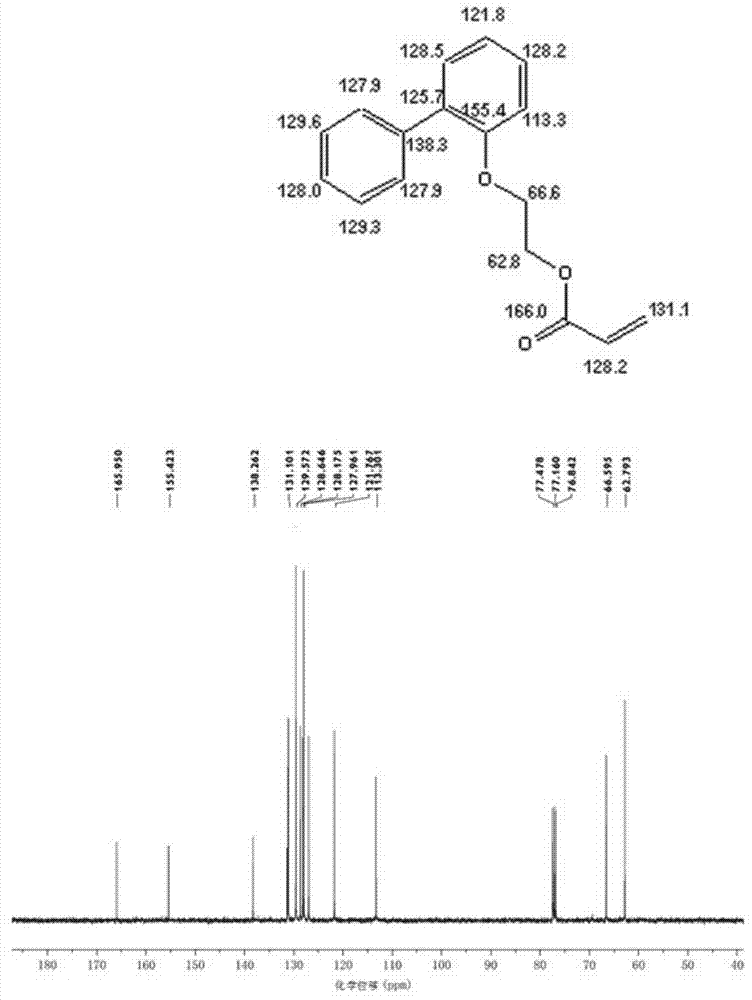
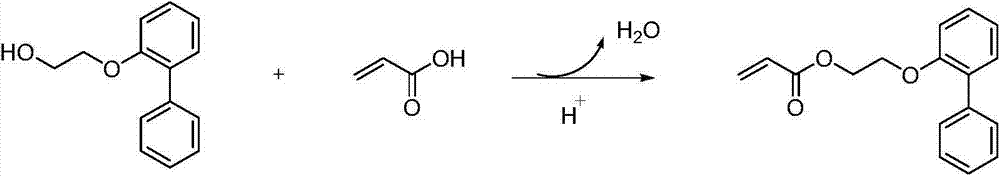
Preparation method of low chroma o-phenyl phenoxyethyl acrylic ester
A technology of o-phenylphenoxyethyl acrylate and low chroma, which is applied in the field of acrylate preparation, can solve the problems of increased production cost and limited effect, and achieve the effects of cost saving, good effect and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 grams of p-hydroxyanisole, 1.84 grams of activated carbon with pH 2-4, nitrogen protection was passed through during the reaction, and the reaction was refluxed at 80-85° C. for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0034] After the reaction was finished, the active carbon was removed by filtration, and the resulting filtrate was washed twice with 5% aqueous sodium hydroxide solution, then washed three times with distilled water, and finally dried with anhydrous magnesium sulfate, and then the polymerization inhibitor p-hydroxybenzene was added to the dried solution. 0.2 gram of methyl ether, at 50 DEG C, under the vacuum degree of 0.1MPa, remove...
Embodiment 2
[0036] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 g of p-hydroxyanisole, 1.84 g of 200-mesh active silica gel, nitrogen protection was passed through during the reaction, and the reaction was refluxed at 80-85° C. for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0037] After the reaction, remove the silica gel by filtration, wash the resulting filtrate twice with 5% aqueous sodium hydroxide solution, then wash three times with distilled water, and finally dry with anhydrous magnesium sulfate, then add the polymerization inhibitor p-hydroxybenzene to the dried solution. 0.2 gram of methyl ether, at 50 DEG C, under the vacuum degree of 0.1MPa condition, remove solvent cyclohexane with rotary evaporator, ...
Embodiment 3
[0039] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 grams of hydroquinone, 3.68 grams of activated carbon with a pH of 2-4, nitrogen protection was passed through during the reaction, and the reaction was refluxed at 80-85° C. for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0040] After the reaction was finished, the active carbon was removed by filtration, and the resulting filtrate was washed twice with 5% aqueous sodium hydroxide solution, then washed three times with distilled water, and finally dried with anhydrous magnesium sulfate, and then the polymerization inhibitor p-hydroxybenzene was added to the dried solution. 0.2 gram of methyl ether, at 50 DEG C, under the vacuum degree of 0.1MPa condit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com