Water-soluble hyperbranched fluorescent polymer and preparation method and applications thereof
A fluorescent polymer, water-soluble technology, applied in the field of hyperbranched polymer synthesis, to achieve the effects of simple synthesis, broad biological application prospects, and simple product purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
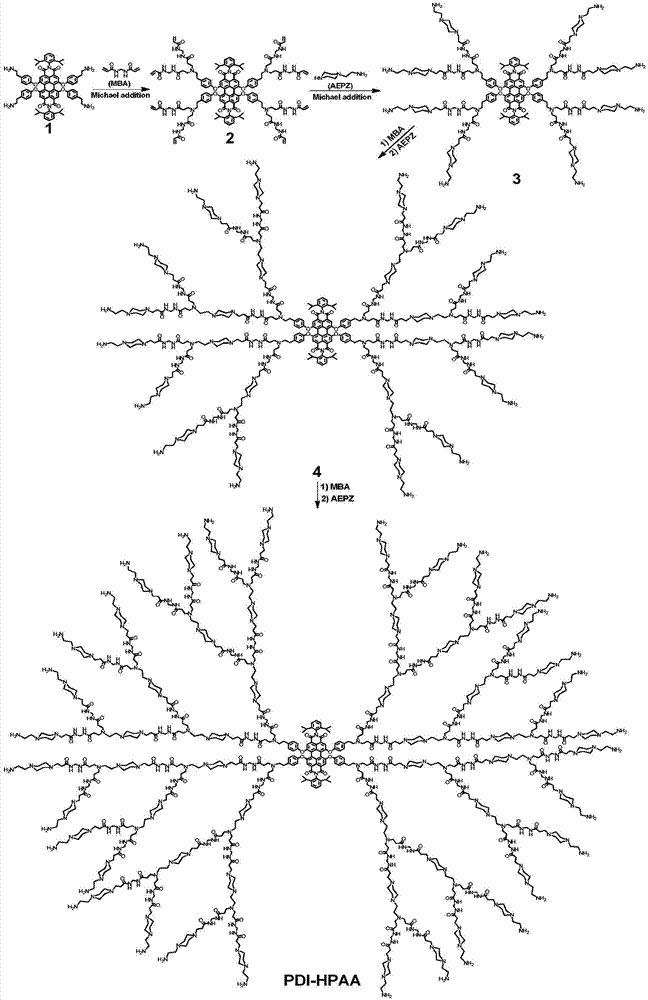
[0039] Example 1 Synthesis of water-soluble hyperbranched fluorescent polymer (PDI-HPAA) by Michael addition method
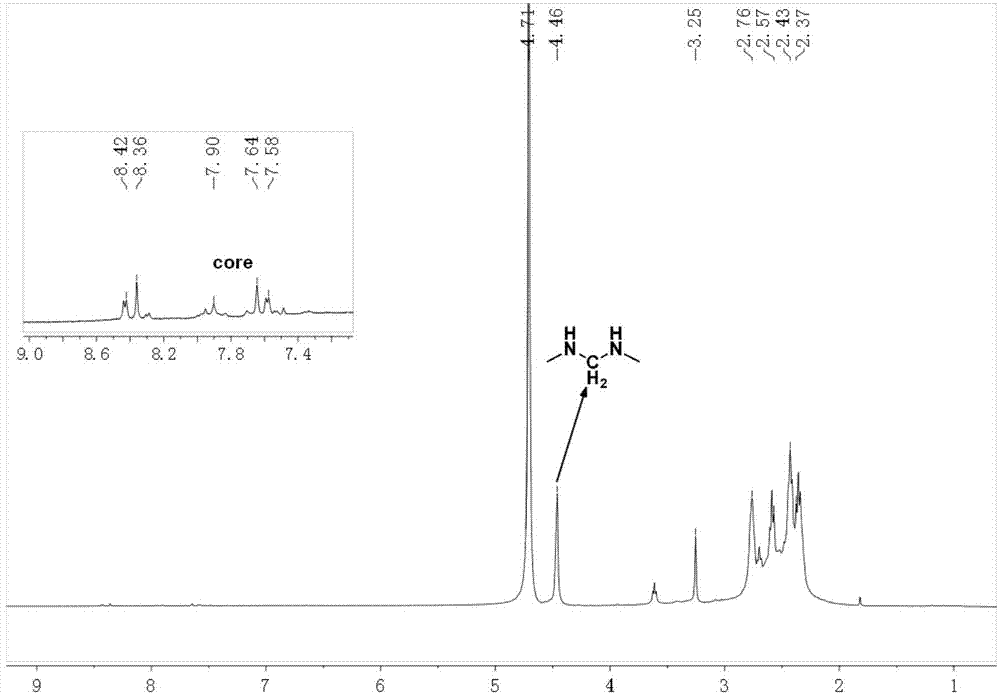
[0040] 1. Add 200.0mg of compound 1 (0.16mmol) and 246.5mg of N,N-methylenebisacrylamide (MBA) (1.6mmol) into 5mL of a mixed solvent of methanol and water with a volume ratio of 4:1, under nitrogen Stir at room temperature for 2 h under atmosphere, then react at 50°C for 50 h, wait for the reaction temperature to drop to room temperature, wash with 50 mL of a mixture of diethyl ether and n-hexane with a volume ratio of 3:1 repeatedly for 3 times, and dry in vacuo to obtain a red solid intermediate product 2. Yield 95%. 1 HNMR(400MHz,DMSO)δ8.62(d,J=51.8Hz,16H),7.94(s,4H),7.43(s,2H),7.25(s,12H),6.98(s,8H),6.17( d,J=45.1Hz,16H),5.59(s,8H),4.45(s,16H),3.38(s,16H),2.70(s,20H),2.24(s,16H),1.10(s,24H ).
[0041] 2. Add 248.5mg of intermediate product 2 (0.1mmol) and 129.0mg of aminoethylpiperazine (APEZ) (1.0mmol) to 6.0mL of a mixed solvent of methanol and water w...
Embodiment 2
[0047] Embodiment 2 live cell uptake experiment
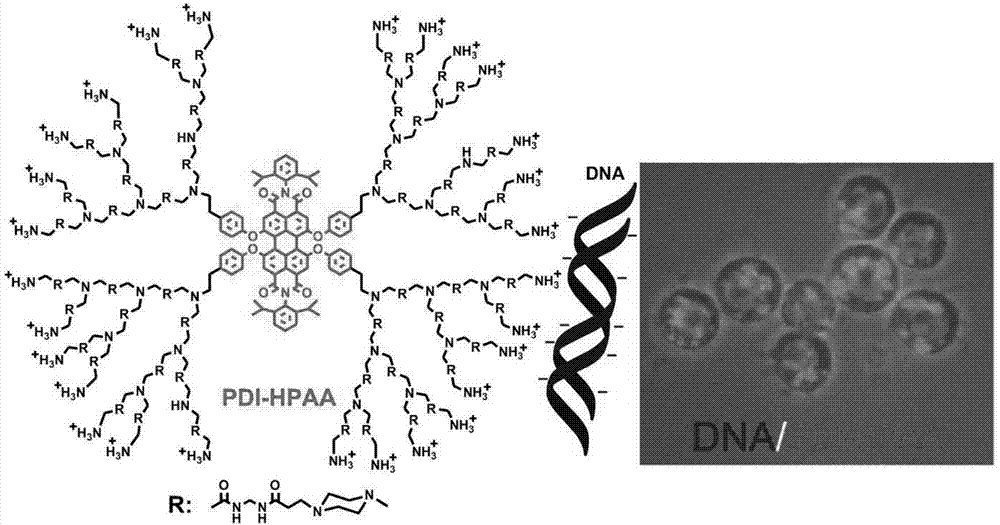
[0048] The water-soluble hyperbranched fluorescent polymer synthesized in Example 1, namely compound 3, compound 4, and PDI-HPAA were incubated with living cells, and it was found that it took a long time for compounds 3 and 4 to enter living cells, while PDI-HPAA could quickly Efficiently enters living cells, exhibits good biocompatibility and low toxicity. 2μM carrier molecule PDI-HPAA can penetrate the cell membrane of living cells and enter the cells after incubation for about 1 hour.
Embodiment 3
[0049] Embodiment 3 gene transfection experiment
[0050] Using the water-soluble hyperbranched fluorescent polymer PDI-HPAA complexed DNA transfected in Example 1 to transfect living cells, the ratio of DNA and PDI-HPAA complexed, that is, the number of ammonium salts at the end of the hyperbranched macromolecule and the ratio of the phosphate radical of the DNA molecule were investigated. Effect of number ratio (N / P) on transfection experiments. By studying the DNA and PDI-HPAA complexes transfected cells with different N / P ratios (2:1, 4:1, 8:1), it was found that the carrier molecule PDI-HPAA was in all N / P ratio conditions All showed high transfection activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com