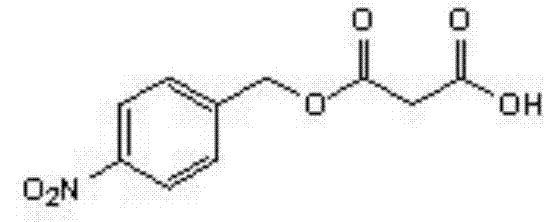
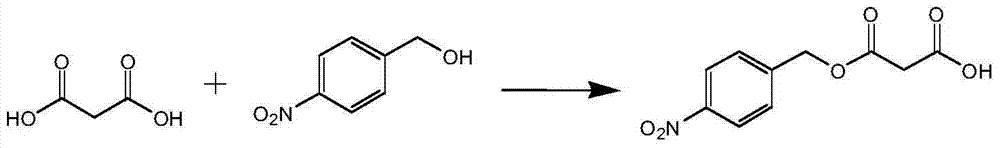
Method for enzymatic synthesis of mono-4-nitrobenzyl malonate
A technology of nitrobenzyl alcohol malonate and p-nitrobenzyl alcohol, which is applied in the field of enzyme-catalyzed synthesis of p-nitrobenzyl alcohol malonate, can solve the problems of low yield, large reaction energy consumption, High raw material costs and other issues, to achieve the effect of high product purity and yield, simple post-processing, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of p-nitrobenzyl alcohol malonate:
[0024] Add 1.53g (0.01mol) of p-nitrobenzyl alcohol, 2.08g (0.02mol) of malonic acid, 40.5ml (0.5mol) of tetrahydrofuran into a 150ml Erlenmeyer flask. Novozymes) 0.1g (0.001mol), add anhydrous CaCl210g (0.09mol), react at 45°C for 48 hours, filter to remove lipase; the reaction liquid is distilled under reduced pressure to remove the solvent, and the obtained residue is added to toluene 40ml and 20% Sodium carbonate solution 100ml, stirred, allowed to stand for stratification, and the separated aqueous phase was acidified with 20% hydrochloric acid until solids were precipitated, filtered under reduced pressure, and washed. The solid product was obtained by drying, and the white product p-nitrobenzyl malonate was obtained, weighing 1.68 g, and the yield was 70.2%.
Embodiment 2
[0026] Synthesis of p-nitrobenzyl alcohol malonate:
[0027] Add 1.53g (0.01mol) of p-nitrobenzyl alcohol, 5.20g (0.05mol) of malonic acid, 10ml (0.1mol) of xylene and acetone (6.3ml of xylene, 3.7ml of acetone) into a 150ml Erlenmeyer flask, and heat in a water bath to 40°C, equilibrate for 20 minutes, add Novozym435 (Novozym, Denmark) 0.8g (0.008mol), add anhydrous MgSO430g, react at 40°C for 24 hours, filter to remove lipase; the reaction solution is distilled off under reduced pressure to obtain Add 40ml of benzene and 80ml of 20% sodium bicarbonate solution to the residue, stir, let stand to separate layers, and acidify the separated aqueous phase with 20% hydrochloric acid until solids are precipitated, filter under reduced pressure, and wash. The solid product was obtained by drying, and the white product p-nitrobenzyl malonate was obtained, weighing 1.73 g, and the yield was 72.5%.
Embodiment 3
[0029] Synthesis of p-nitrobenzyl alcohol malonate:
[0030] Add 1.53g (0.01mol) of p-nitrobenzyl alcohol, 1.25g (0.01mol) of sodium malonate, 27ml (0.24mol) of xylene and tetrahydrofuran (22.2ml of xylene, 4.8ml of tetrahydrofuran) into a 150ml Erlenmeyer flask , heated in a water bath to 30°C, equilibrated for 20 minutes, added Candida sp.Lipase (Beijing New Century Kaitai Biotechnology Co., Ltd.) 0.1g (0.01mol), reacted at 30°C for 10 hours, filtered to remove the lipase; the reaction solution was distilled off under reduced pressure Solvent, add 40ml of toluene and 100ml of 20% sodium carbonate solution to the obtained residue, stir, let stand to separate layers, and acidify the separated aqueous phase with 20% hydrochloric acid until solid precipitates, filter under reduced pressure, and wash. The solid product was obtained by drying, and the white product, p-nitrobenzyl malonate, weighed 2.04 g, and the yield was 85.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com