Preparation method of linezolid derivative
A technology of linezolid and derivatives, applied in the field of medicine, can solve problems such as being difficult to obtain, and achieve the effects of low production cost, strong operability, and easy analysis and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
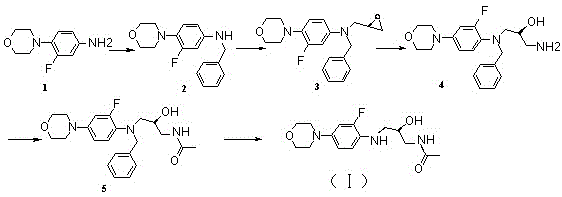
[0030] Example 1 Preparation of Linezolid Derivatives (I)
[0031] like figure 1 Shown, the preparation of intermediate 2: Take linezolid intermediate 1 (10 g, 0.051 mol) and dissolve in dichloromethane (100 ml), add triethylamine (10.29 g, 0.101 mol) under ice-cooling, then Then add benzyl bromide (13g, 0.075 mol) to the reaction solution obtained and reflux at 45°C for 24 hours, point plate to observe that about half of the raw material remains in the reaction, add 50ml saturated sodium bicarbonate to the reaction solution, and separate the organic phase. The aqueous phase was extracted with dichloromethane again, and the combined organic phase was concentrated to obtain 15 g of oil, which was then further purified by silica gel column chromatography, and the eluent was petroleum ether:ethyl acetate=95:5 to obtain 5.4 g of yellow intermediate Body 2 is solid.
[0032] Intermediate 2 1 H NMR (300 MHz, CDCl3) data is:
[0033] 1H NMR (300 MHz, CDCl3) δ: 7.27~7.35 (m, 5H), ...
Embodiment 2
[0044] Example 2 Preparation of Linezolid Derivatives (I)
[0045] like figure 1 As shown, the preparation of intermediate 2: Dissolve linezolid intermediate 1 (10 g, 0.051 mol) in dichloromethane (100 ml), add under ice-cooling, pyridine (0.201 mol), and then add benzyl bromide (0.101 mol) of the reaction solution obtained was refluxed at 45°C for 24 hours, and the plate was observed to observe that about half of the raw materials remained in the reaction, and 50ml of saturated sodium bicarbonate was added to the reaction solution, and the organic phase was separated, and the aqueous phase was then washed with dichloromethane Methane extraction, the combined organic phases were concentrated to obtain 15 g of oil, which was further separated and purified by silica gel column chromatography, the eluent was petroleum ether:ethyl acetate=95:5, and 5.4 g of yellow intermediate 2 was obtained as a solid.
[0046] Intermediate 2 1 H NMR (300 MHz, CDCl3) data is:
[0047] 1H NMR ...
Embodiment 3
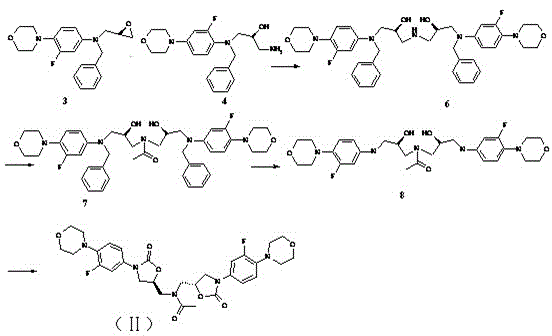
[0058] Example 3 Preparation of Linezolid Derivatives (II)
[0059] like figure 2 As shown, the preparation of intermediate 6: Take intermediate 3 (2.74g, 0.008mol) and intermediate 4 (2.88g, 0.008mol) prepared in Example 1 and react in isopropanol (55 ml) at 80°C for 16h , Spot the plate to observe that there are about 20% of the raw material remaining, and the crude product obtained by concentrating the reaction solution is purified through the column, and the developing solvent is dichloromethane:methanol=98:2~90:10, and 2.5 grams of the intermediate 6 product is obtained.
[0060] Preparation of Intermediate 7: Dissolve Intermediate 6 (2.5g, 0.003mol) in dichloromethane (30 ml), add pyridine (0.56g, 0.007mol), acid anhydride (0.36g, 0.003mol), and react at room temperature After 2 hours, the plate reaction was almost complete, water (50 ml) was added, dichloromethane was used for extraction, the combined organic phase was evaporated to dryness, and the column was purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com