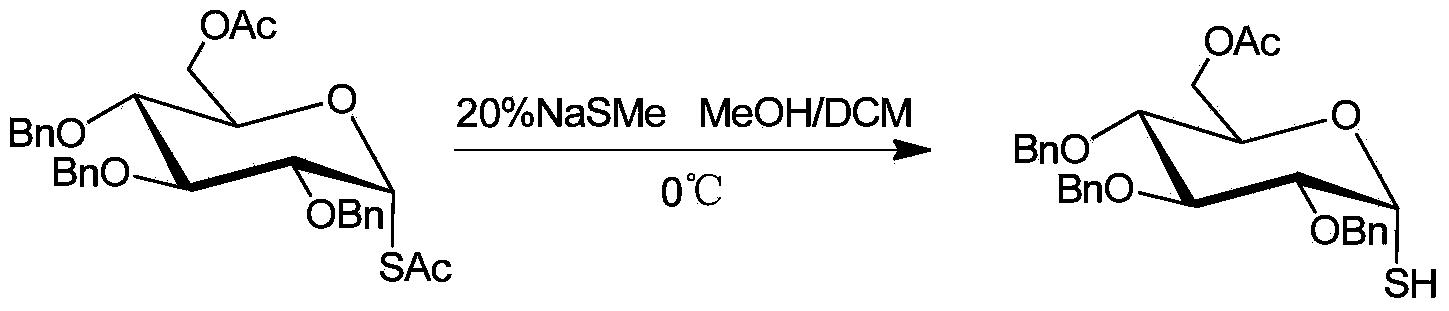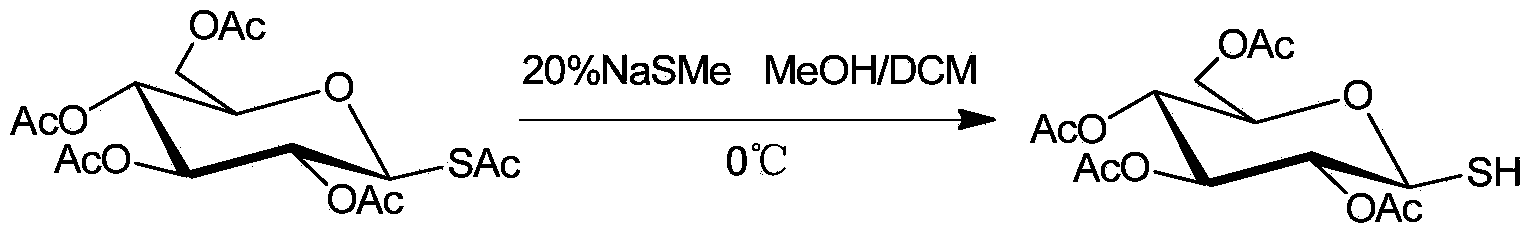Method of selectively removing sulphur acetyl protecting group in saccharide
A thioacetyl and selective technology, applied in the field of organic chemical synthesis, can solve the problems of narrow trial range of deprotected substrates, difficulty in controlling the reaction process, difficult storage of reagents, etc., and achieve cheap reagents, easy separation and fast speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Selective desulfurization of 6-O-acetyl-2,3,4-tri-O-benzyl-1-S-acetyl-α-D-glucose
[0017] The reaction formula is:
[0018]
[0019] Under ice-bath conditions, add 0.55g (1mmol) 6-O-acetyl-2,3,4-tri-O-benzyl-1-S-acetyl-α-D-glucose to a 50 ml round bottom flask , 9mL methanol, dichloromethane solvent, wherein the volume ratio of methanol and dichloromethane is 1:2, stirred for 10min, 1eq aqueous solution of sodium methyl mercaptide was added dropwise to the flask, and TLC tracked the plate. After the reaction was completed, 10% hydrochloric acid was added to the reaction flask to quench the reaction. Add an appropriate amount of saturated saline and ethyl acetate, extract and separate the liquids, combine the organic phases, and dry over anhydrous sodium sulfate; concentrate under reduced pressure, and the residue is quickly separated by column chromatography to obtain 0.46 g of the product with a yield of 90%. 1 H NMR (400MHz, CDCl 3 )δ7.37-7.26(m,15H)...
Embodiment 2
[0020] Example 2: Selective desulfurization of 2,3,4,6-tetra-O-acetyl-1-S-acetyl-β-D-glucose
[0021] The reaction formula is:
[0022]
[0023] Under ice bath conditions, add 0.41g (1mmol) 2,3,4,6-tetra-O-acetyl-1-S-acetyl-β-D-glucose, 9mL methanol, di Chloromethane solvent, wherein the volume ratio of methanol and dichloromethane is 1:2, stirred for 10min, 1eq sodium methyl mercaptide aqueous solution was added dropwise to the flask, and TLC followed the spot plate. After the reaction was completed, 10% hydrochloric acid was added to the reaction bottle to quench the reaction. Add an appropriate amount of saturated saline and ethyl acetate, extract and separate the liquids, combine the organic phases, and dry over anhydrous sodium sulfate; concentrate under reduced pressure, and the residue is quickly separated by column chromatography to obtain 0.32 g of the product with a yield of 88%. 1 H NMR (400MHz, CDCl 3 )δ5.19(t,J=9.4Hz,1H),5.10(t,J=9.7Hz,1H),4.97(t,J=9.5Hz,1H)...
Embodiment 3
[0024] Example 3: 2,3,4,6-tetra-O-acetyl-1-S-acetyl-β-D-galactose selective desulfurization of acetyl
[0025] The reaction formula is:
[0026]
[0027] Under ice-bath condition, add 0.41g (1mmol) 2,3,4,6-tetra-O-acetyl-1-S-acetyl-β-D-galactose, 9mL methanol, Dichloromethane solvent, wherein the volume ratio of methanol and dichloromethane is 1:2, stirred for 10min, 1eq sodium methyl mercaptide aqueous solution was added dropwise to the flask, and TLC tracked the spot plate. After the reaction was completed, 10% hydrochloric acid was added to the reaction flask to quench the reaction. Add an appropriate amount of saturated saline and ethyl acetate, extract and separate the liquids, combine the organic phases, and dry over anhydrous sodium sulfate; concentrate under reduced pressure, and the residue is quickly separated by column chromatography to obtain 0.35 g of the product with a yield of 95%. 1 H NMR (600MHz, CDCl 3 )δ5.44(d,J=3.2Hz,1H),5.19(t,J=9.9Hz,1H),5.03(dd,J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com