Fusion tag protein
A fusion tag and fusion protein technology, applied in the field of molecular biology, can solve problems such as expression inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Obtaining of wild-type HREV107 (1-125) gene fragment and construction of C89S mutant
[0034] The DNA fragment of wild-type HREV107 (1-125) can be obtained by PCR amplification reaction using a human liver cDNA library (purchased from Wuhan Sanying Biotechnology Co., Ltd.) as a template.
[0035] Using the human liver cDNA library as a template and using NdeI-REV-N / REV-XhoI-C as primers, PCR amplification was performed to obtain wild-type HREV107(1-125) with NdeI and XhoI restriction sites at both ends. Gene fragment. The fragment was inserted into the pET-21a vector through these two restriction sites to obtain the pET-21a-HREV107N-His plasmid. The primer sequences are as follows:
[0036] NdeI-REV-N: 5'-GGAATTC CATATG CGTGCGCCCATTCCA-3' (SEQ ID No: 4)
[0037] REV-XhoI-C: 5'-CCG CTCGAG GGCGACTCCATAGCGCAG-3' (SEQ ID No: 5)
[0038] Among them, CATATG and CTCGAG are NdeI and XhoI restriction enzyme cutting sites, respectively.
[0039] Since the 89th...
Embodiment 2
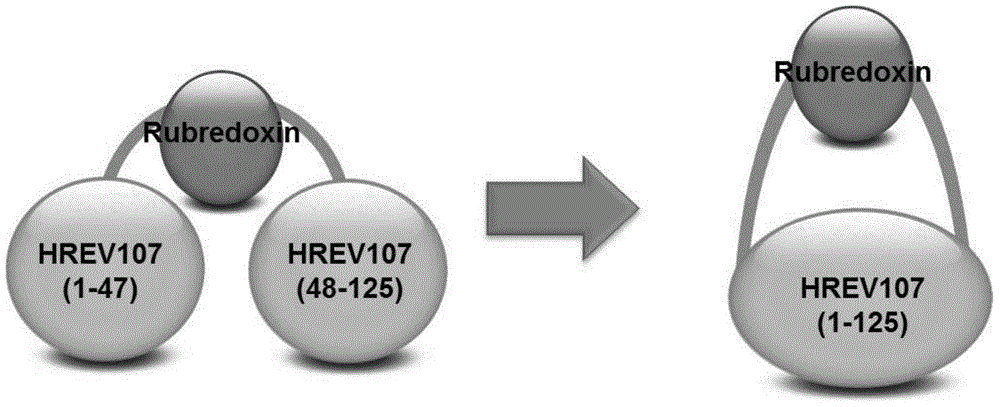
[0045] Example 2: Plasmid construction, expression and characterization of Hrevn-rubredoxin-Hrevc-His fusion protein
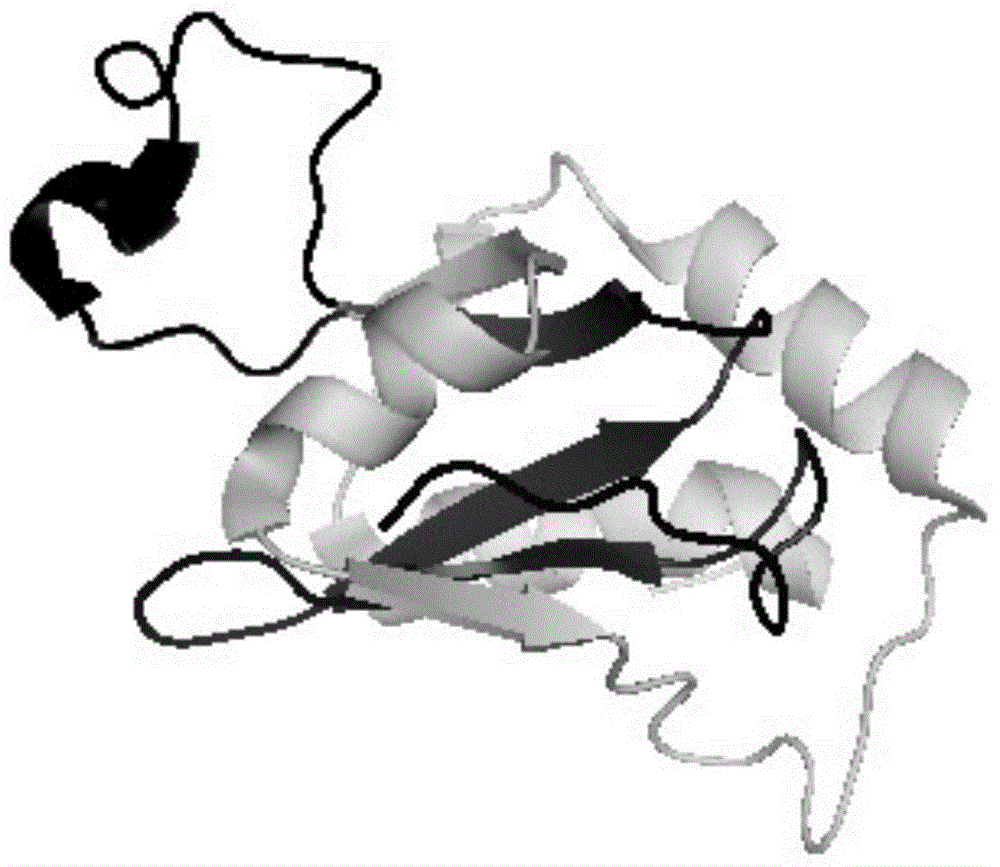
[0046] In this example, rubredoxin protein was used as the target protein, and it was inserted into the P38-K57 region of HREV107N.
[0047] The rubredoxin protein used in this example and the linker and enzyme cleavage site selected during the construction process are only used to illustrate the construction method of the novel fusion tag protein, and do not limit the scope of application of the present invention.
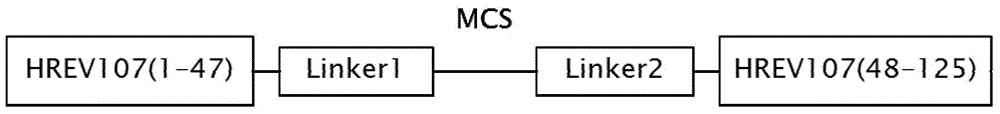
[0048] 1) Construction of recombinant plasmids
[0049] The recombinant plasmid expression region includes the following 6 parts from A to F from the 5' end to the 3' end, and the sequences are as follows:
[0050] A) HREV107(1-47): refer to the 1st-141th nucleotide sequence of SEQ ID No: 2 in the sequence listing, which encodes the 1st-47th amino acid residues of SEQ ID No: 1;
[0051] B) Linker1:
[0052] 5' ccatgg ggtggttctggtggtggttctggtc...
Embodiment 3
[0067] Example 3: Construction and testing of a novel fusion protein expression system
[0068] In this example, four different proteins were used to test the effect of the novel fusion protein expression system.
[0069] 1) Introduction of the target protein used in the test
[0070] AID: AID (auto-inhibiting domain) is a part of the α subunit of AMP-activated protein kinase (AMPK). AMPK is an important protein kinase, which is expressed in many organs and tissues of the human body, including liver, brain, and skeletal muscle. The increase in the ratio of AMP / ATP in organisms can activate the activity of AMPK. After being activated, AMPK can promote a series of catabolic processes in multicellular organisms, and at the same time inhibit some anabolic pathways, such as the biosynthesis of lipids, proteins and carbohydrates. Thereby maintaining the ratio of AMP to ATP at a constant level. AMPK is therefore a key molecule in bioenergy metabolism.
[0071] C-clamp: Wnt signal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com