IL-2 and MART-1 dual-gene co-expression recombinant vector as well as preparation method and application of recombinant vector
A technology of MART-1 and IL-2, which is applied in the field of recombinant vector and its preparation, can solve the problems of immune regulation, low anti-tumor ability, lack of targeting of immunotherapy, etc., and achieve enhanced amplification ability and cell viability, Improve the effect of melanoma immunotherapy and expand the effect of anti-tumor immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Obtaining IL-2 Gene Fragments Containing Specific Restriction Sites
[0071] 1. Primer design
[0072] According to the nucleotide sequence of the IL-2 gene (as shown in SEQ ID NO:1 in the sequence listing) and the expected insertion multiple cloning site on the pIRES2-EGFP plasmid vector, design specific primers as follows:
[0073] IL-2 upstream primer (as shown in SEQ ID NO:4 in the sequence listing):
[0074] 5'-CCG GAATTC ATGTACAGGATGCAACTCC-3' (the underlined part is the EcoR I restriction site sequence), IL-2 downstream primer (as shown in SEQ ID NO:5 in the sequence listing):
[0075] 5'-CGC GGATCC TCAAGTCAGTGTTGAGATGATGC-3' (the underlined part is the restriction site sequence of BamH I).
[0076] 2. Obtain cDNA template
[0077]TRIzon method was used to extract RNA from CIK cells (Cytokine-Induced Killer, cytokine-induced killer cells) or mononuclear cells isolated from human peripheral blood (TRIzon total RNA extraction kit was purchased fro...
Embodiment 2
[0084] Example 2: Construction of pIRES2-IL-2-EGFP recombinant vector
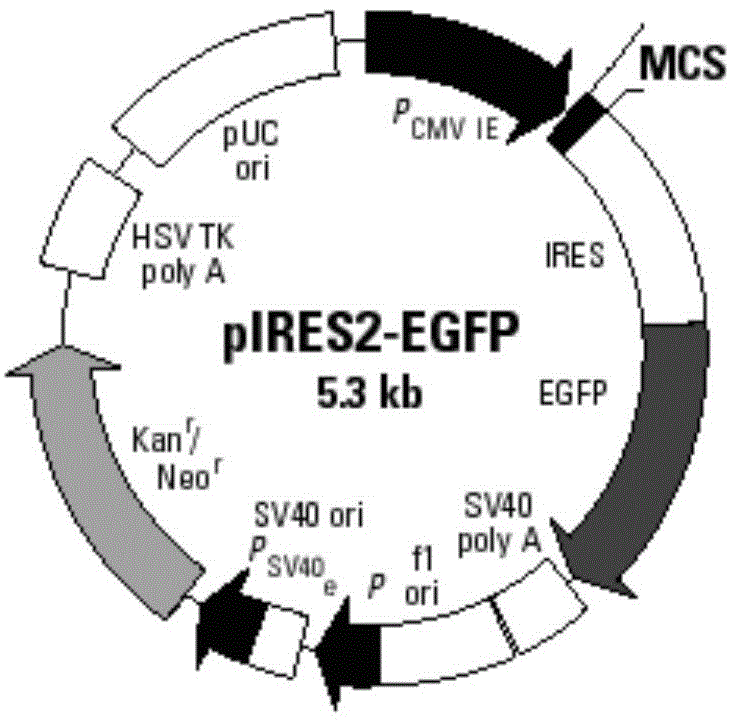
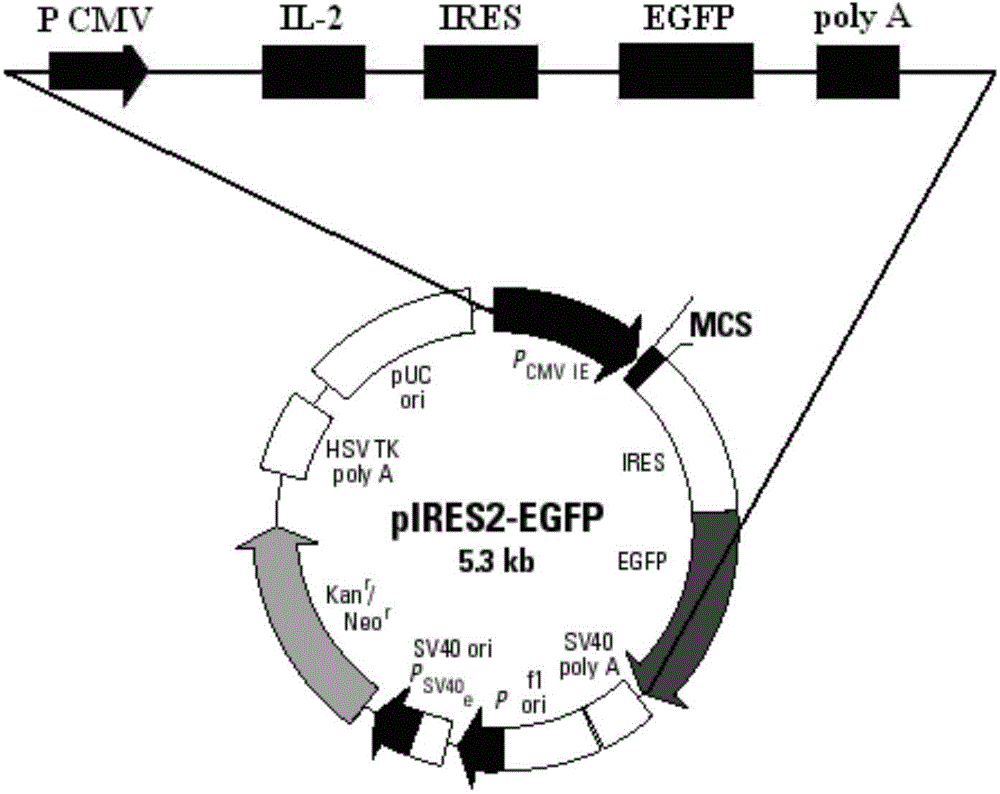
[0085] Using restriction endonucleases EcoR I and BamH I, digest the pIRES2-EGFP plasmid (the multiple cloning site of the plasmid contains EcoR I and BamH I restriction sites) and the IL-2 gene fragment obtained in Example 1, respectively , to obtain the linearized pIRES2-EGFP vector and the IL-2 gene sequence after digestion; use T4 DNA ligase system for ligation reaction, incubate at 22°C for 30 minutes, and then inactivate at 70°C for 5 minutes, Construct the pIRES2-IL-2-EGFP recombinant vector (such as image 3 shown).
[0086] Structural features of the pIRES2-EGFP plasmid (eg figure 2 shown), it can be seen that after the IL-2 gene is inserted into the multiple cloning site of the pIRES2-EGFP plasmid, it is located upstream of the self-sequence IRES of the plasmid vector (such as image 3 shown), that is, IL-2 and EGFP sequences were expressed separately under the same promoter.
[0087] 1. Dou...
Embodiment 3
[0113] Example 3: Double PCR method to obtain the MART-1 gene fragment with restriction endonuclease cohesive ends
[0114] According to the MART-1 gene sequence and the expected insertion multiple cloning site on the pIRES2-EGFP plasmid vector, two pairs of primers with different lengths and sticky ends of restriction endonucleases were designed; RNA extracted from melanoma cell A375 was reversed The transcribed cDNA was used as a template, and the above two pairs of primers were used for PCR amplification to obtain two PCR amplification products; the two PCR amplification products were mixed and then denatured and annealed sequentially to obtain four MART-1 gene fragments , where the two ends of the two MART-1 gene fragments have restriction endonuclease cohesive ends, so that the MART-1 gene fragments can be directionally connected to the plasmid vector without restriction endonuclease digestion. in the multiple cloning site.
[0115] Compared with the traditional PCR prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com