Phage drug protein display system and application thereof
A protein display and phage technology, applied in the field of biomedicine, can solve the problems of increased secretion of immunosuppressive cytokines, cancer cell recognition, and reduced killing ability, achieving short amplification cycle, enhanced anti-tumor immune response, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1. Nucleic acid vector construction
[0067] Use the NCBI database to search the human PD-1 gene sequence, select the coding gene of the extracellular segment of PD-1 protein (aa21-170), and optimize the sequence according to the codon preference of E. coli. Technology Co., Ltd. for gene synthesis.
[0068] Design the upstream and downstream primers P1 and P2 for gene amplification of the extracellular region of PD-1 protein:
[0069] P1: GCCATGGCCCAGGTGCAGCTGCCGGGTTGGTTCCTGGACTCTCCG
[0070] P2: CTGATATCTTTGGATCCAGCGGCCGCACCAACCAGGGTCTGGAACTGACCAG
[0071] Using the synthetic PD-1 gene as a template, the target gene was amplified by PCR using Super-Fidelity DNA polymerase. The PCR reaction system is: 25μL buffer, 20μL ddH 2 O, 1 μL dNTP, 1 μL primer P1, 1 μL primer P2, 1 μL template, 1 μL high-fidelity DNA polymerase. The PCR reaction program was: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 20 s, annealing at 60°C for 30 s, extension at 72...
Embodiment 2
[0074] Example 2. Construction of PD-1 Display Phage
[0075] Inoculate 500 μL of the ER2738 strain carrying the pSEX81-PD-1 plasmid into 50 mL of 2YT medium (containing 100 μg / mL ampicillin and 100 mM glucose), and culture with shaking at 220 rpm at 37°C until OD600=0.5. Add M13KO7ΔPⅢ helper phage (the ratio of the number of Escherichia coli to the number of phages is 20:1) and mix well, put it in a 37°C incubator and culture it for 20 minutes, then place it in a shaker at 37°C at 220rpm and shake it for 45 minutes to make Escherichia coli obtain kanamycin resistance. Pour the bacterial solution into a centrifuge tube and centrifuge at 2000g for 10min at 4°C, discard the supernatant, add 50mL of fresh 2YT medium (containing 100μg / mL ampicillin, 50μg / mL kanamycin) to resuspend the bacteria, shake at 37°C Shake culture at 220rpm in the bed overnight.
[0076] Collect the bacterial solution and centrifuge at 2000g for 10min at 4°C, filter the supernatant with a 0.22μm sterile ...
Embodiment 3
[0080] Example 3.PD-1 Displays Binding Specificity of Phage
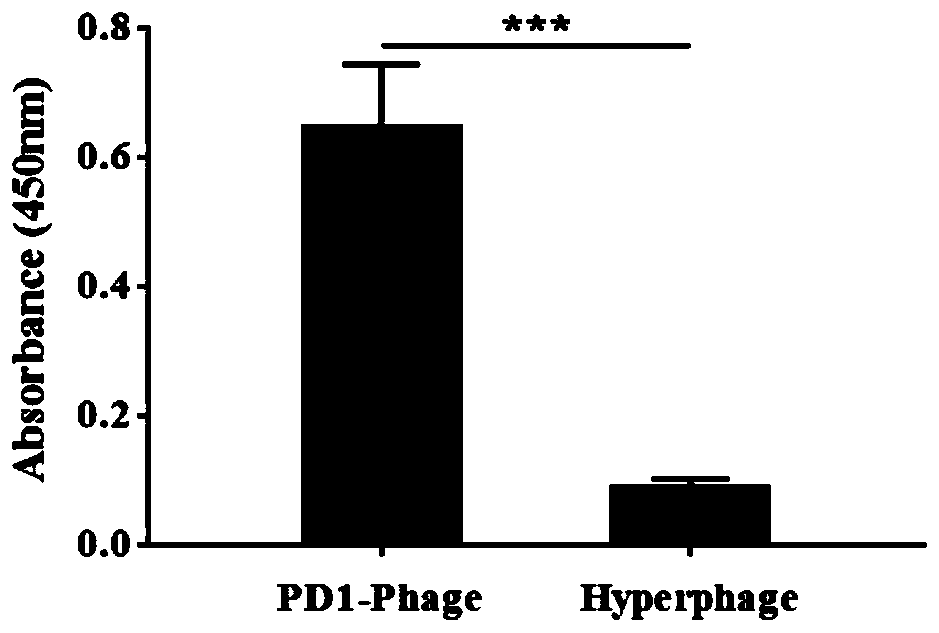
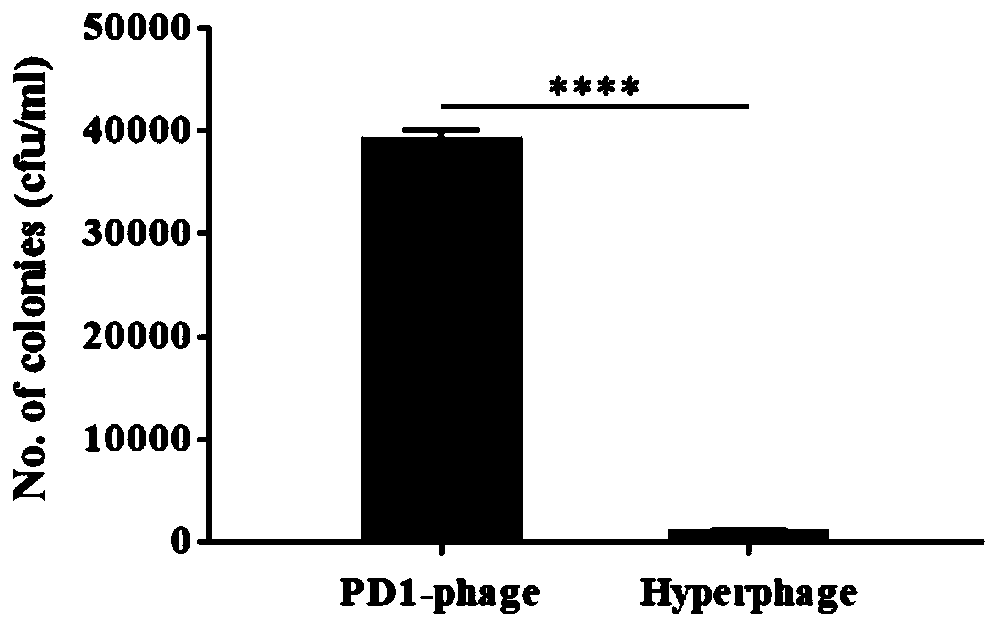
[0081] with dilution buffer (100mM NaHCO 3 , pH 8.6) Dilute the PD-1 displaying phage and helper phage to 10 10 pfu / mL, 100 μL each was added to the wells of a 96-well microtiter plate, and incubated overnight in a 4°C refrigerator. TBST (PBS+0.5% Tween 20) was washed 5 times, and 200 μL of blocking solution (TBST containing 5% BSA) was added to each well to block at 37° C. for 1 hour. Wash 5 times with TBST, dilute rhPD-L1 to 1 μg / mL, add 100 μL to each well and incubate at 37 °C for 1 h. After washing 5 times with TBST, the mouse anti-PD-L1 monoclonal antibody was diluted with TBST at a ratio of 1:2000, and 100 μL was added to each well and incubated at 37°C for 1 h. After washing 5 times with TBST, the HRP-labeled goat anti-mouse secondary antibody was diluted with TBST at a ratio of 1:5000, and 100 μL was added to each well and incubated at 37°C for 1 h. Wash 5 times with TBST, add 100 μL TMB ELISA chromogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com