Novel water-based preparation formulation of methylamino abamectin benzoate
A technology of methylamino abamectin and benzoate, applied in the directions of insecticides, biocides, animal repellents, etc., can solve the problem of no delayed effect, volatilization and loss of active ingredients, and insufficient rapid effect of harmful organisms and other problems, to increase the utilization rate, increase the content of active ingredients, and achieve the effects of contact killing and stomach poisoning.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
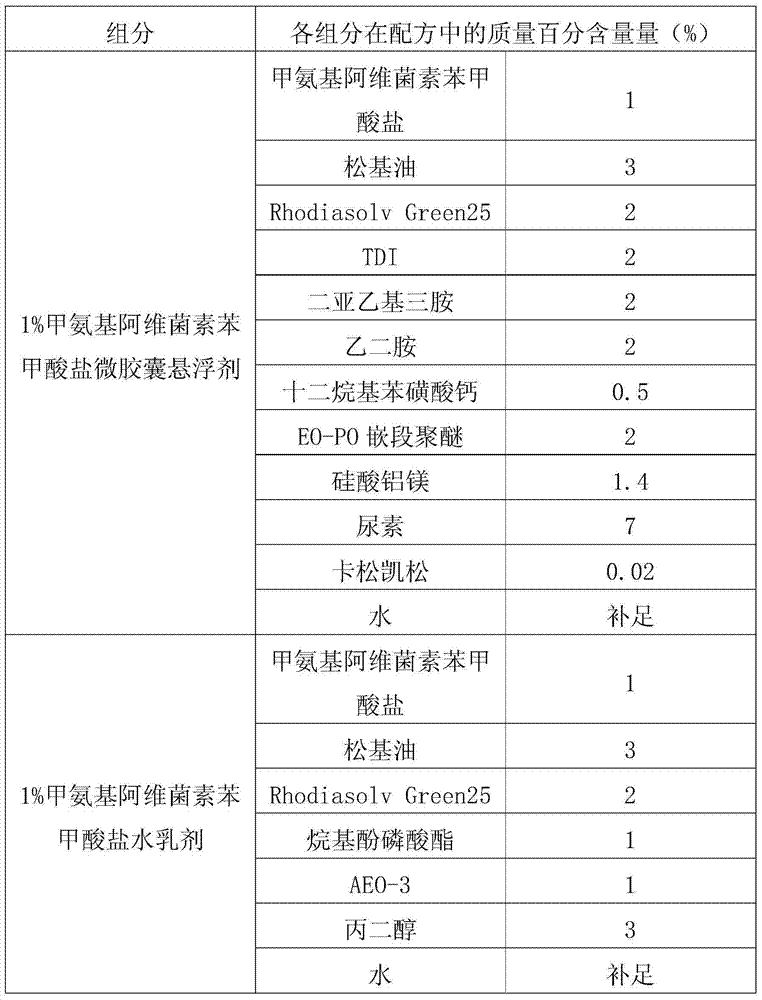
[0031] The processing method of emamectin benzoate new dosage form ZW in the present embodiment is as follows: first adopt interfacial polymerization method to process 1% emamectin benzoate microcapsule suspension, conventional method processes 1% Emamectin benzoate emulsion in water, the two are evenly mixed according to the mass ratio of 7:3 to obtain 1% emamectin benzoate ZW.
[0032]Table 1, interfacial polymerization method processing emamectin benzoate microcapsule suspension and the formula of conventional method processing water emulsion.
[0033]
[0034] The prepared 1% emamectin benzoate new dosage form ZW can meet the following technical indicators: suspension rate ≥ 93%; emulsion stability qualified; cyst formation rate ≥ 91%; automatic dispersibility ≥ 90%; The pH value is qualified; all indexes of cold storage are qualified; all indexes of hot storage are qualified; all indexes of freeze-thaw stability are qualified.
Embodiment 2
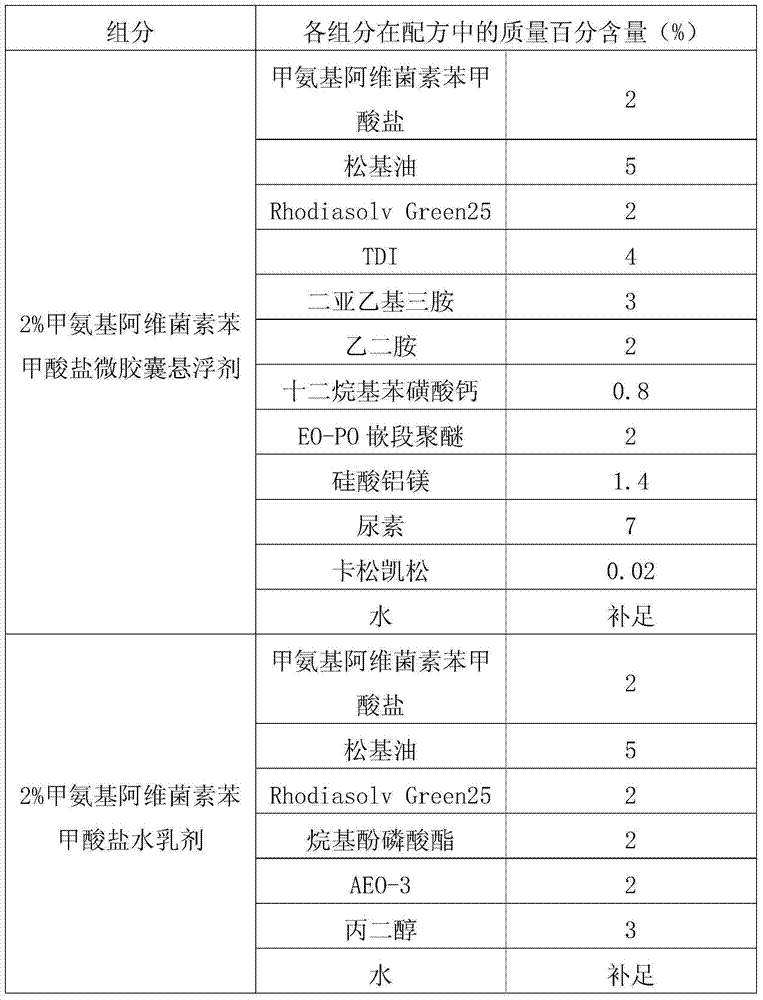
[0036] The processing method of emamectin benzoate new dosage form ZW in the present embodiment is as follows: first adopt interface polymerization method to process 2% emamectin benzoate microcapsule suspension concentrate, conventional method processes 2% Emamectin benzoate emulsion in water, the two are evenly mixed according to the mass ratio of 7:3 to obtain 2% emamectin benzoate ZW.
[0037] Table 2, interfacial polymerization method processing emamectin benzoate microcapsule suspension and the formula of conventional method processing water emulsion.
[0038]
[0039] The prepared 2% emamectin benzoate new dosage form ZW can meet the following technical indicators: suspension rate ≥ 93%; emulsion stability qualified; cyst formation rate ≥ 91%; automatic dispersibility ≥ 90%; The pH value is qualified; all indexes of cold storage are qualified; all indexes of hot storage are qualified; all indexes of freeze-thaw stability are qualified.
Embodiment 3
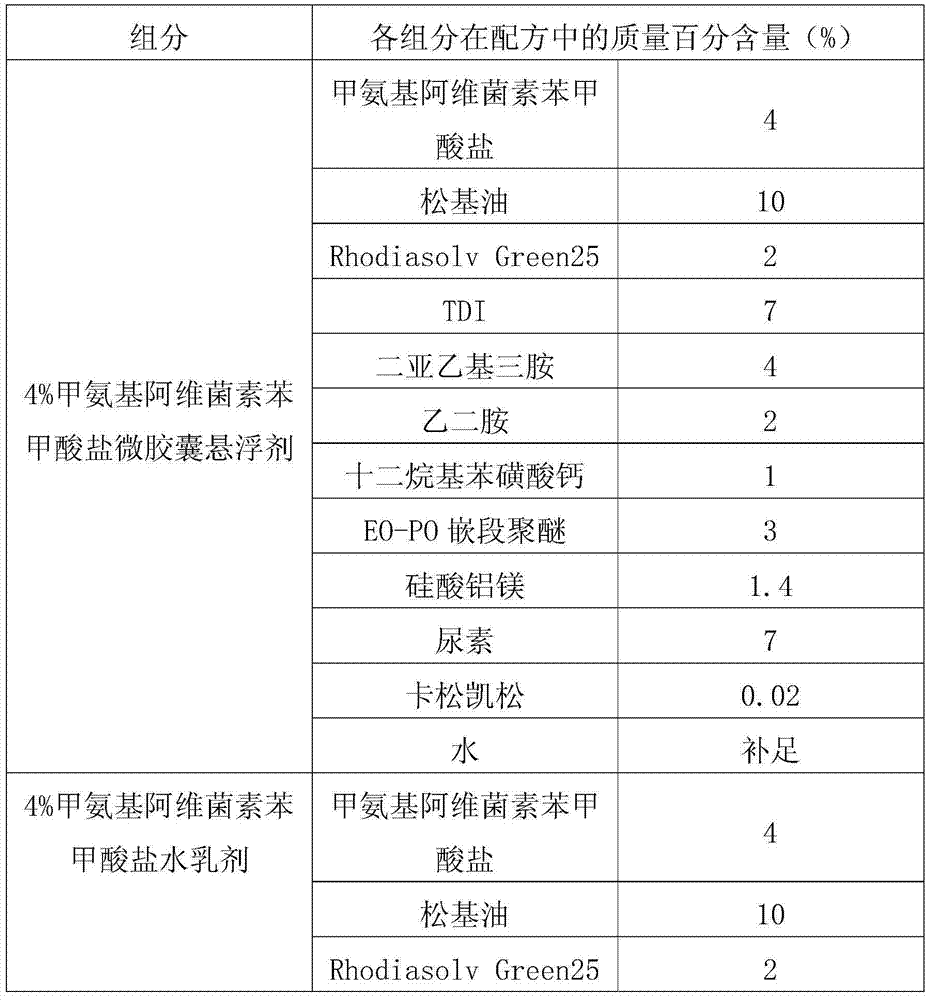
[0041] In the present embodiment, the processing method of emamectin benzoate new dosage form ZW is as follows: firstly adopt the interfacial polymerization method to process 4% emamectin benzoate microcapsule suspension, and conventional method processes 4% Emamectin benzoate emulsion in water, the two are evenly mixed according to the mass ratio of 7:3 to obtain 4% emamectin benzoate ZW.
[0042] Table 3, interfacial polymerization processing emamectin benzoate microcapsule suspension and conventional method processing water emulsion mixed.
[0043]
[0044]
[0045] The prepared 4% emamectin benzoate new dosage form ZW can meet the following technical indicators: suspension rate ≥ 93%; emulsion stability qualified; cyst formation rate ≥ 91%; automatic dispersibility ≥ 90%; The pH value is qualified; all indexes of cold storage are qualified; all indexes of hot storage are qualified; all indexes of freeze-thaw stability are qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com