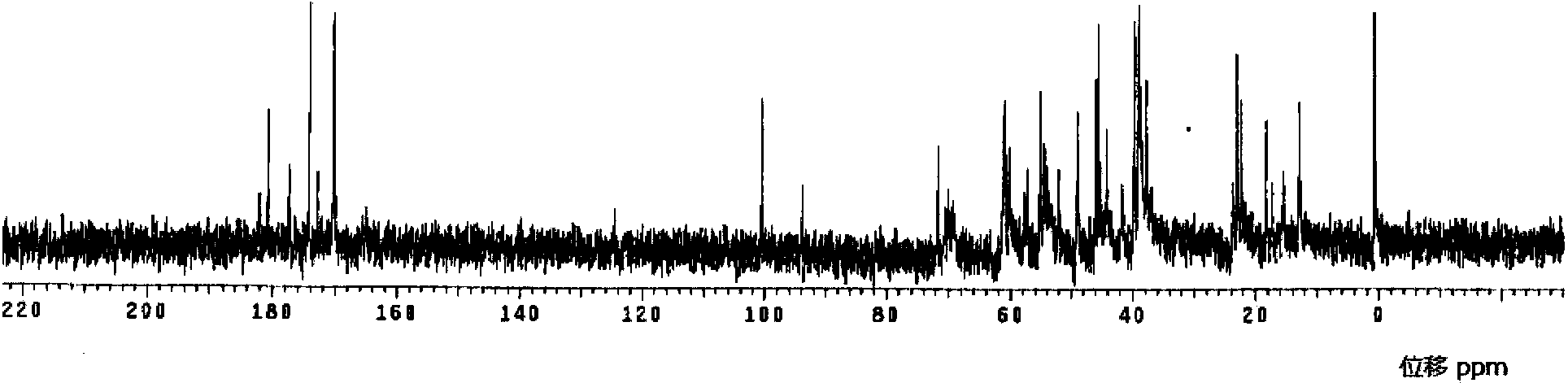
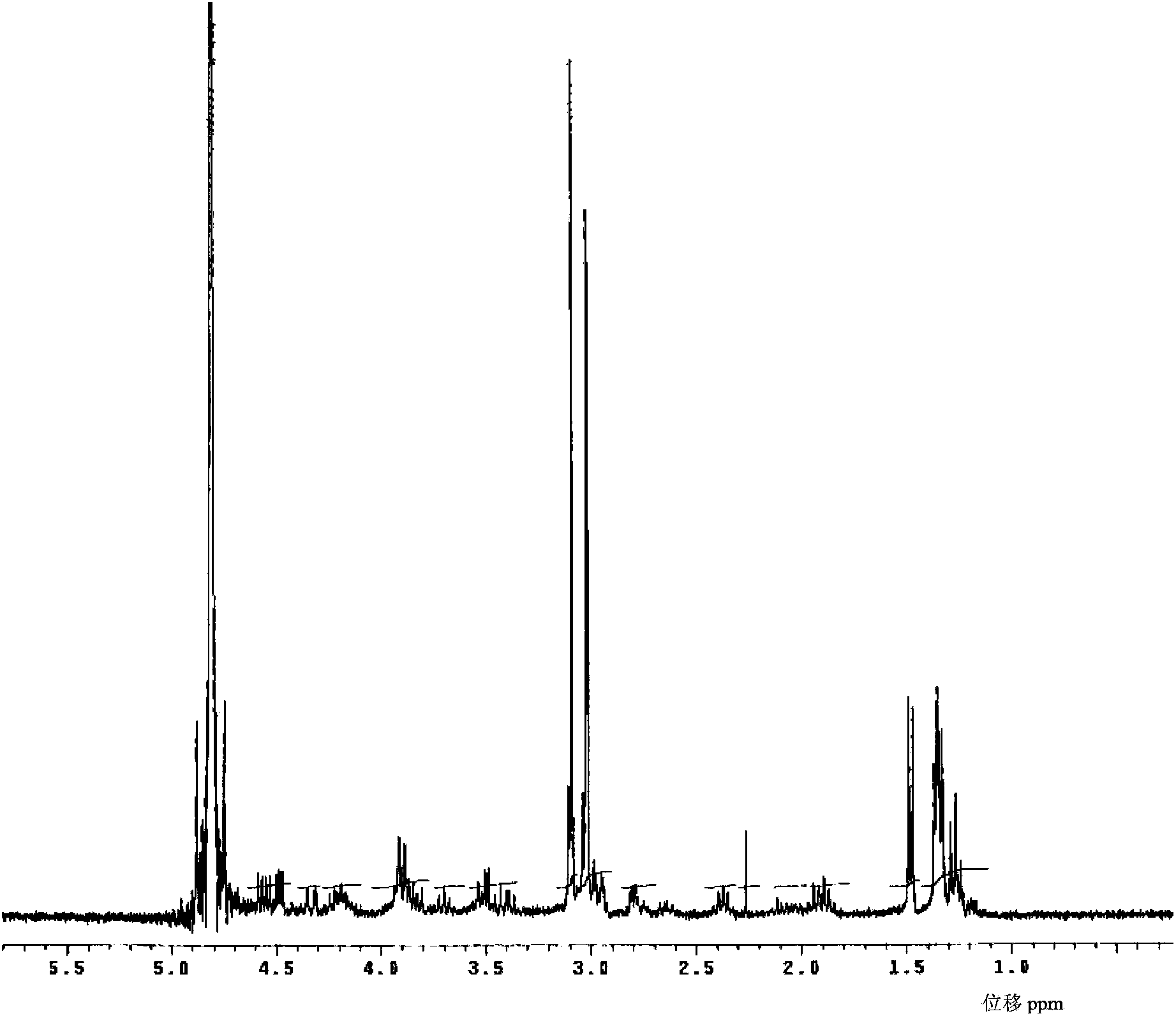
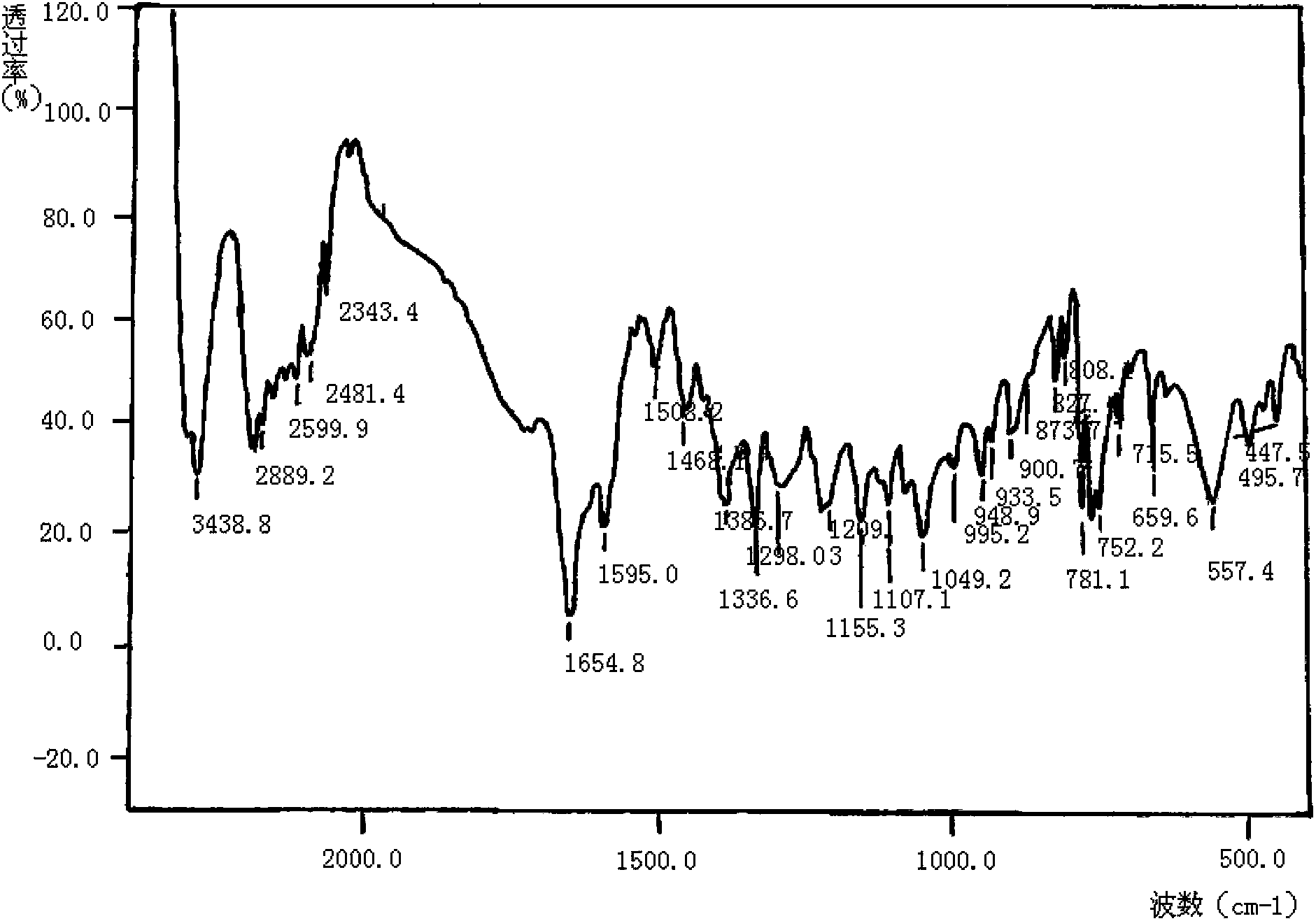
Meropenem raw medicine, preparation method thereof and pharmaceutical composition containing same
A technology of meropenem and raw materials, which is applied in the fields of pharmaceutical formulations, antibacterial drugs, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Add 2600g of pure water into a 5L flask, stir and heat up to 65°C, add 200g of crude meropenem into the flask, dissolve within 2 minutes, then cool down to 20°C within 3 minutes, add 20g of medical activated carbon for 2-3 minutes to decolorize , filter at 20°C, add the filtrate to a 10L three-necked flask, add 0.2g of seed crystals, keep the stirring speed at about 120 rpm, cool down to 5°C, start to calculate the crystal growth time, keep the temperature at 2-3°C, 10 Hours later, add 5200ml of acetone pre-cooled to 0-5°C dropwise, grow crystals at 2-3°C for 30 minutes after dripping, filter, rinse the filter cake with acetone, and dry the filter cake under vacuum at 30°C for 6 hours to obtain the meropenem raw material drug 176 grams, yield 88%.
[0095] Among them, the content of meropenem is 99.04% (calculated as anhydrous matter); impurity A in related substances is 0.10%; impurity B is 0.11%; any unknown single impurity is 0.02%; the sum of other impurities except...
Embodiment 2
[0134] Add 320g of pure water into a 500ml flask, stir and heat up to 50°C, add 20g of crude meropenem into the flask, dissolve within 2 minutes, then cool down to 10°C within 2 minutes, add 0.2g of medical activated carbon for 2-3 minutes to decolorize , filter at 10°C, add the filtrate to a 1000ml three-neck flask, add 0.4g seed crystals, then cool down to 5°C, start to calculate the crystal growth time, keep the stirring speed at 110 rpm, and the temperature at 3-5°C, and grow the crystals 8 hours, then add dropwise 480ml of acetone pre-cooled to 0-5°C, control the temperature of the filtrate II at 0°C-5°C, grow the crystal for 1 hour after dropping, filter, rinse the filter cake with acetone, and vacuum the filter cake at 20°C After drying for 5 hours, 17.2 g of meropenem bulk drug was obtained with a yield of 86%.
[0135] Among them, the content of meropenem is 100.04% (calculated as anhydrous matter); impurity A in related substances is 0.08%; impurity B is 0.09%; any u...
Embodiment 3
[0139] Add 200g of pure water into a 500ml flask, stir and raise the temperature to 70°C, quickly add 20g of crude meropenem into the flask, dissolve it within 2 minutes, then quickly cool down to 30°C within 2 minutes, add 4g of medical activated carbon for decolorization 5 Minutes, filter at 30°C, add the filtrate to a 1000ml three-necked flask, cool down to 20°C, add 2g of seed crystals, then cool down to 5°C, start to calculate the crystal growth time, keep the stirring speed at 150 rpm, and the temperature at 3-5 After growing the crystal for 2.1 hours at ℃, add 100ml of acetone dropwise, grow the crystal for 30 minutes after dropping, filter, rinse the filter cake with acetone, and dry the filter cake in vacuum at 40°C for more than 2 hours to obtain 16.6 g of meropenem raw material drug, yield 83%.
[0140] Among them, the content of meropenem is 99.7% (calculated as anhydrous matter); impurity A in related substances is 0.08%; impurity B is 0.13%; any unknown single im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com