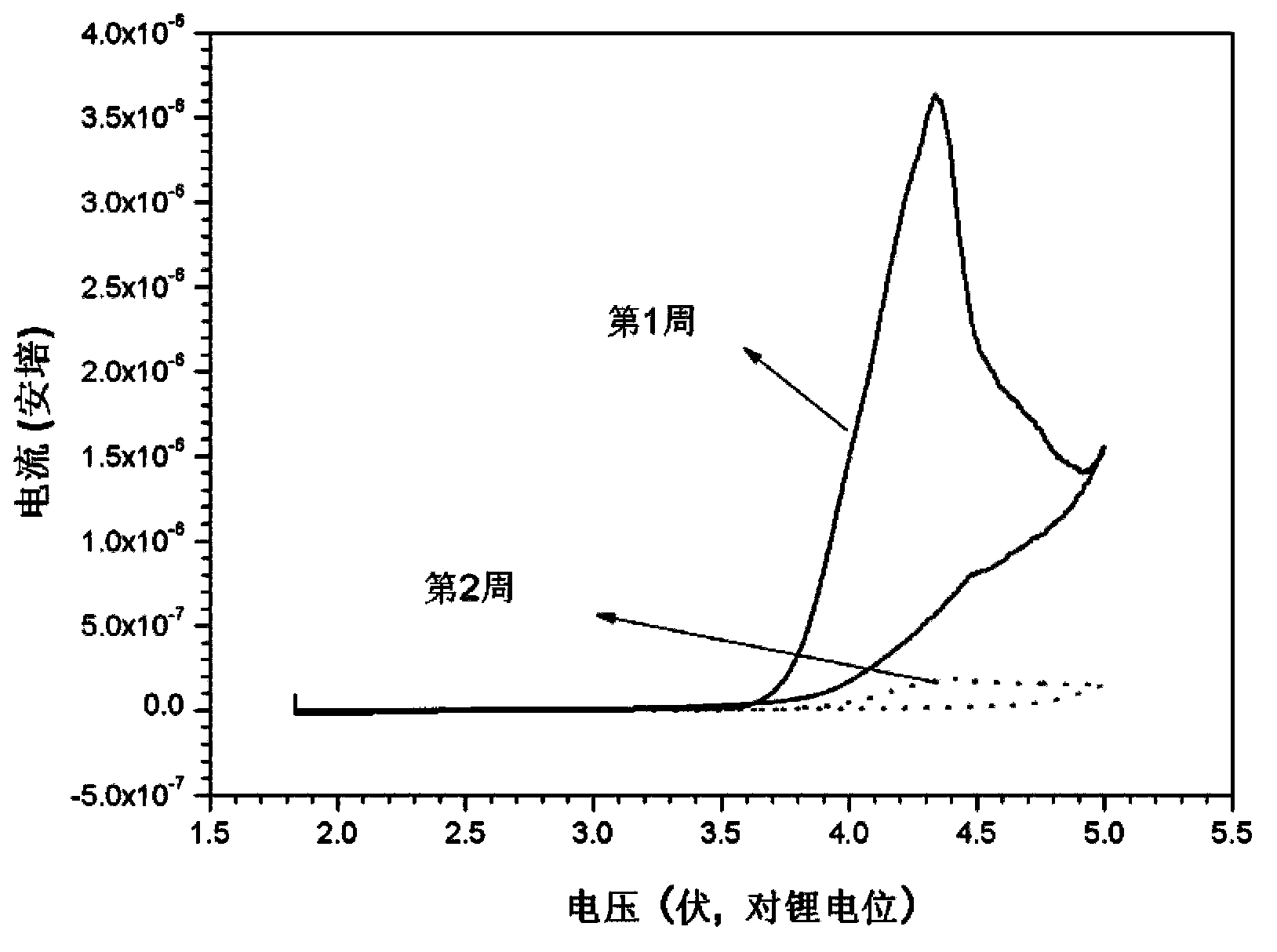
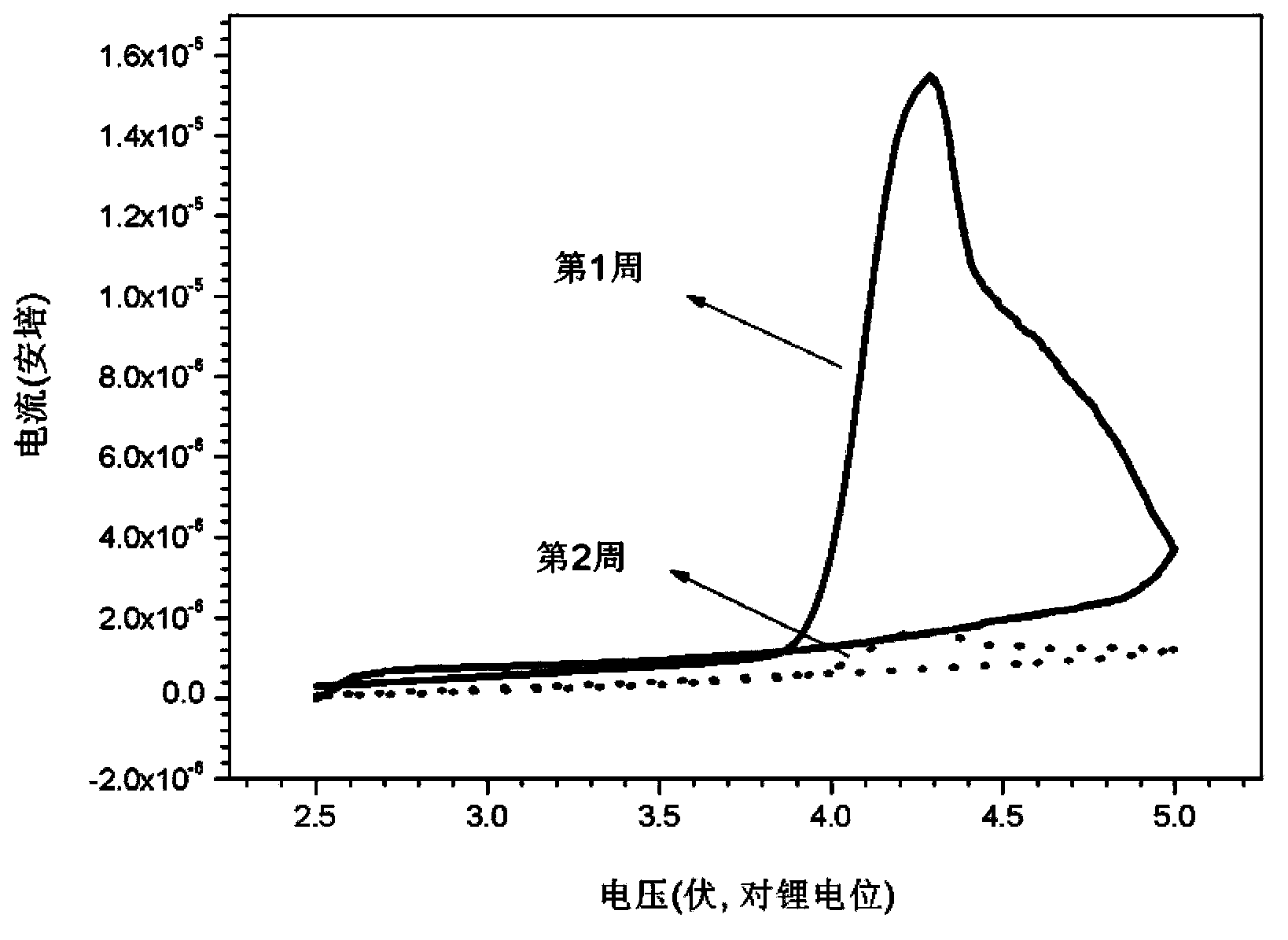
Electrolyte and secondary lithium battery and capacitor containing electrolyte
An electrolyte and capacitor technology, applied in the field of secondary lithium batteries and capacitors, can solve the problems of current collector corrosion, limit the wide application of electrolyte, affect the cycle life and efficiency of secondary lithium batteries and electrochemical capacitors, and achieve enhanced phase capacitive and improve cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The present embodiment provides a kind of electrolytic solution, comprises:
[0049] An organic solvent consisting of dimethyl carbonate (DMC) and ethylene carbonate (EC) in a volume ratio of 1:1;
[0050] Conductive lithium salt consisting of LiFSI at a concentration of 0.01 mol / L;
[0051] Anti-corrosion additive consisting of MTES at a concentration of 1×10 -6 mol / liter.
[0052] This electrolyte solution is denoted as A1, and its performance will be described in detail below.
Embodiment 2
[0054] The present embodiment provides a kind of electrolytic solution, comprises:
[0055] An organic solvent, consisting of dimethyl carbonate and ethylene carbonate at a volume ratio of 1:1;
[0056] Conductive lithium salt consisting of LiFSI at a concentration of 1 mol / l;
[0057] Anti-corrosion additive consisting of MTES at a concentration of 1×10 -6 mole / liter;
[0058]The second lithium salt (that is, another lithium salt that coexists with LiFSI in the electrolyte at the same time, and this lithium salt does not have any negative effects on the electrochemical performance of LiFSI), consists of bis(trifluoromethylsulfonyl) Lithium amide (LiTFSI) at a concentration of 0.4 mol / L.
[0059] The electrolyte solution is referred to as A2. The difference between A2 and A1 lies in the concentration of LiFSI and the addition of a second lithium salt LiTFSI. The performance of A2 will be described in detail below.
Embodiment 3
[0061] The present embodiment provides a kind of electrolytic solution, comprises:
[0062] An organic solvent, consisting of dimethyl carbonate and ethylene carbonate at a volume ratio of 1:1;
[0063] Conductive lithium salt consisting of LiFSI at a concentration of 1 mol / l;
[0064] Anti-corrosion additive, consisting of MTES, at a concentration of 0.01 mol / l.
[0065] The electrolyte is referred to as A3. The difference between A3 and A1 lies in the concentration of LiFSI and MTES, and its performance will be described in detail below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com