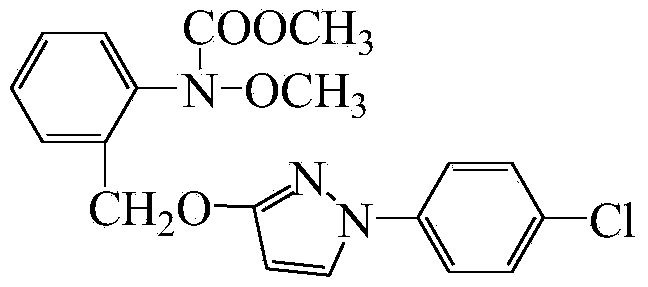Preparation method of 1-(4-chlorophenyl)-3-hydroxypyrazole
A technology of chlorophenyl and pyrazol, which is applied in the field of preparation of 1--3-pyrazol, can solve the problems of uneconomical, high cost, raw material preservation and harsh operating conditions, and achieves reduction of labor and product quality. Good, stable and easy-to-preserve raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
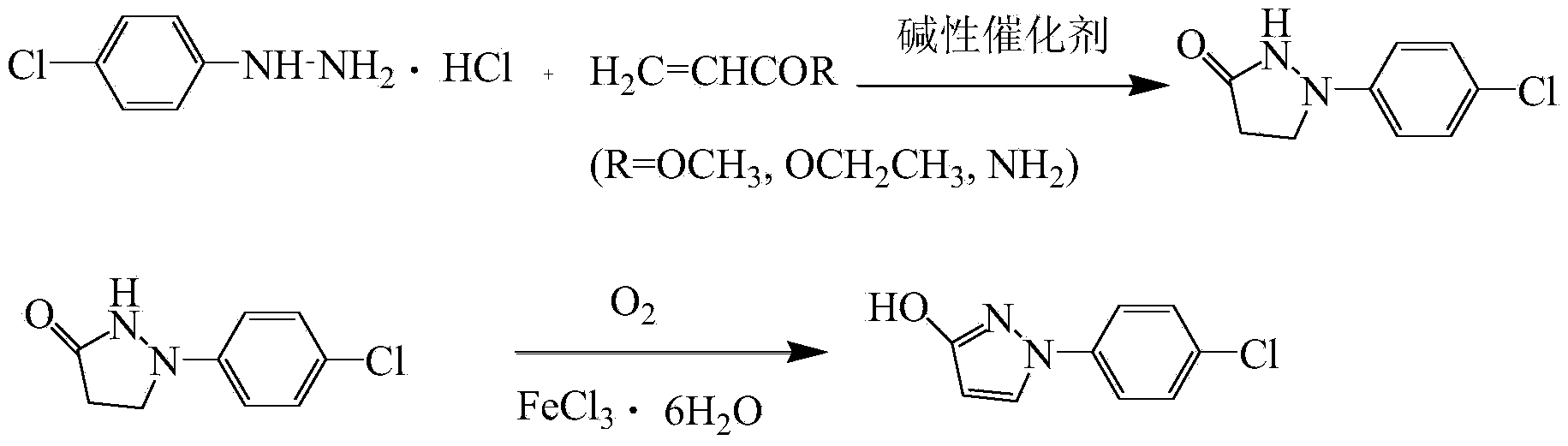
[0025] Add 36.16g (99%, 0.2mol) of p-chlorophenylhydrazine hydrochloride and 216.96g of toluene at room temperature, mix well, add 21.82g (99%, 0.4mol) of sodium methoxide, and dropwise add ethyl acrylate 26.53g at 50°C g (98%, 0.26mol), the dropwise addition was completed in 1 hour; the temperature was raised to 80°C for reflux and stirred for 6 hours, and through liquid phase detection, the main raw material p-chlorophenylhydrazine hydrochloride peak disappeared, that is, the reaction was complete; Continue steaming for 0.5h until no toluene flows out, add 145g of water to dissolve, then add 5.46g (99%, 0.02mol) of ferric chloride hexahydrate, heat up to 50°C and pass air (the flow rate of air is 70L / min) to oxidize 16h until the 1-(4-chlorophenyl)pyrazolidin-3-one peak disappears through the liquid phase detection, that is, the reaction is complete; add 50g of water, cool down to 40°C, add hydrochloric acid dropwise until pH=1, cool down to 30°C , Suction filtration to obta...
Embodiment 2
[0027] Add 36.16g (99%, 0.2mol) of p-chlorophenylhydrazine hydrochloride and 216.96g ethanol at room temperature, mix well, add 99.73g (30%, 0.44mol) of 30wt% sodium ethylate in ethanol, and drop Add 24.49 g (98%, 0.24 mol) of ethyl acrylate, and drop it for 1 hour; raise the temperature to 80° C. and reflux and stir for 6 hours. Through liquid phase detection, the main raw material p-chlorophenylhydrazine hydrochloride peak disappears, that is, the reaction is complete; Distill under reduced pressure, steam until no ethanol flows out and then continue to steam for 0.5h, add 145g of water to dissolve, then add 5.46g (99%, 0.02mol) of ferric chloride hexahydrate, heat up to 60°C and ventilate the air (the flow rate of the air is 70L / min) to oxidize for 12h until the peak of 1-(4-chlorophenyl)pyrazolidin-3-one disappears through liquid phase detection, that is, the reaction is complete; add 50g of water, cool down to 42°C, add hydrochloric acid dropwise until pH=1 , cooled to 30...
Embodiment 3
[0029]Add 36.16g (99%, 0.2mol) of p-chlorophenylhydrazine hydrochloride and 216.96g of methanol at room temperature. After mixing evenly, add 26.18g (99%, 0.48mol) of sodium methoxide, and dropwise add 24.57g of methyl acrylate at 40°C. g (98%, 0.28mol), the dropwise addition was completed in 1 hour; the temperature was raised to 65°C for reflux and stirred for 6 hours, and through liquid phase detection, the peak of the main raw material p-chlorophenylhydrazine hydrochloride disappeared, that is, the reaction was complete; Continue steaming for 0.5h until no methanol flows out, add 145g of water to dissolve, then add 5.46g (99%, 0.02mol) of ferric chloride hexahydrate, heat up to 70°C and pass air (the flow rate of air is 70L / min) to oxidize 10h until the 1-(4-chlorophenyl)pyrazolidin-3-one peak disappears through the liquid phase detection, that is, the reaction is complete; add 50g of water, cool down to 44°C, add hydrochloric acid dropwise until pH=1, cool down to 30°C , S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com