Application of ruthenium complex in antitumor drug preparation
A technology of anti-tumor drugs and ruthenium complexes, which is applied in the field of anti-tumor drugs, can solve the problems of poor recognition ability between normal cells and tumor cells, failure of treatment and treatment, toxic and side effects of tumor patients, etc., and achieve good killing effect in vivo and in vitro Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Mitochondria targeting
[0029] Take the HeLa cells in the logarithmic growth phase and adjust the concentration to 1 × 10 4 / mL, inoculate into 35mm cell culture dish, when the cells grow to 60% confluence, add 10 μM ruthenium complex and mitochondria-specific fluorescent probe Mito-Tracker, incubate for 15 minutes, use a Zeiss inverted fluorescence microscope (Zeiss Axio Observer D1 ) to detect the intracellular localization of ruthenium complexes, the experimental results are as follows figure 1 As shown (A is the bright field, B is the ruthenium complex (red fluorescence), C is the mitochondria-specific fluorescent probe (green fluorescence), D is the overlay of B and C). Experiments show that, figure 1 -D shows that the red fluorescence emitted by the ruthenium complex and the green fluorescence emitted by the mitochondria-specific fluorescent probe are superimposed to present orange fluorescence, which proves that the ruthenium complex can be targeted ...
Embodiment 2
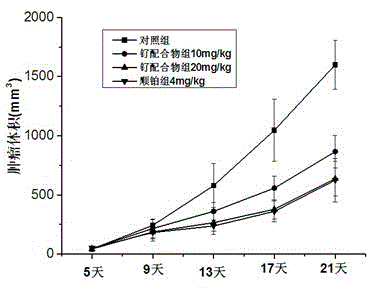
[0030] Example 2 Determination of antitumor activity of ruthenium complexes in vitro
[0031] The screened cell lines include: tumor cells HeLa, A-549, BEL-7402, HepG2, MCF-7, and cisplatin-resistant strain CP / R-A54. The determination adopts bromide tetrazolium blue (MTT) method, its principle is: bromide tetrazolium blue [MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] is A dye that can accept hydrogen atoms. The dehydrogenase associated with NADP in the mitochondria of living cells can convert yellow MTT into insoluble blue-purple formazan in cells, while dead cells have no such function. After dissolving formazan with dimethyl sulfoxide (DMSO), measure the optical density value with a microplate reader at a certain wavelength, and the survival rate of the cells can be quantitatively measured. Experimental procedure: Take HeLa cells in the logarithmic growth phase and adjust the cell density to 5x10 4 cells / mL, inoculated in 96-well culture plate, 200 m...
Embodiment 3
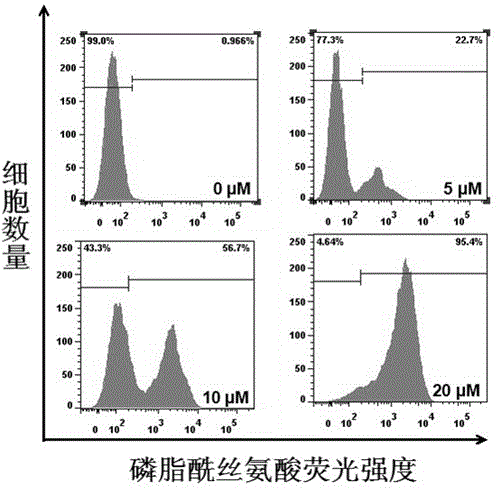
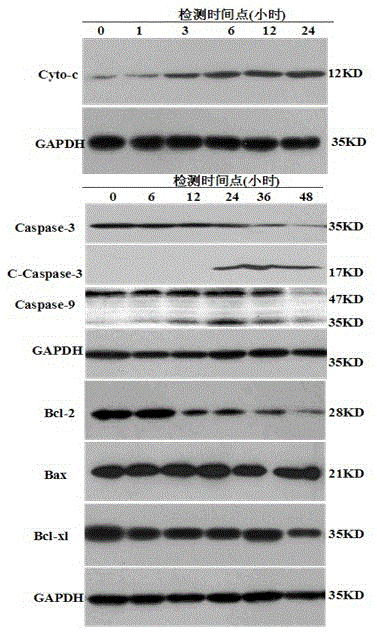
[0036] Example 3 Ruthenium complexes induce tumor cell apoptosis
[0037] The measurement uses flow cytometry Annexin-V staining method, the principle of which is: Phosphatidylserine (Phosphatidylserine, PS) is normally located on the inner side of the cell membrane, but in the early stage of apoptosis, PS can flip from the inner side of the cell membrane to the surface of the cell membrane, exposed to the extracellular environment. Annexin-V is a Ca 2+ Dependent phospholipid-binding protein, which can specifically bind to PS with high affinity. Annexin-V was labeled with fluorescein (FITC), and the labeled Annexin-V was used as a fluorescent probe to detect the occurrence of apoptosis by flow cytometry.
[0038] Experimental procedure: HeLa cells in the logarithmic growth phase were treated with 5 x 10 4 Cells / mL were seeded in 6-well plates, 2 mL per well, placed at 37°C, 5% CO 2 After culturing in a saturated humidity incubator for 24 hours, different concentrations of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com