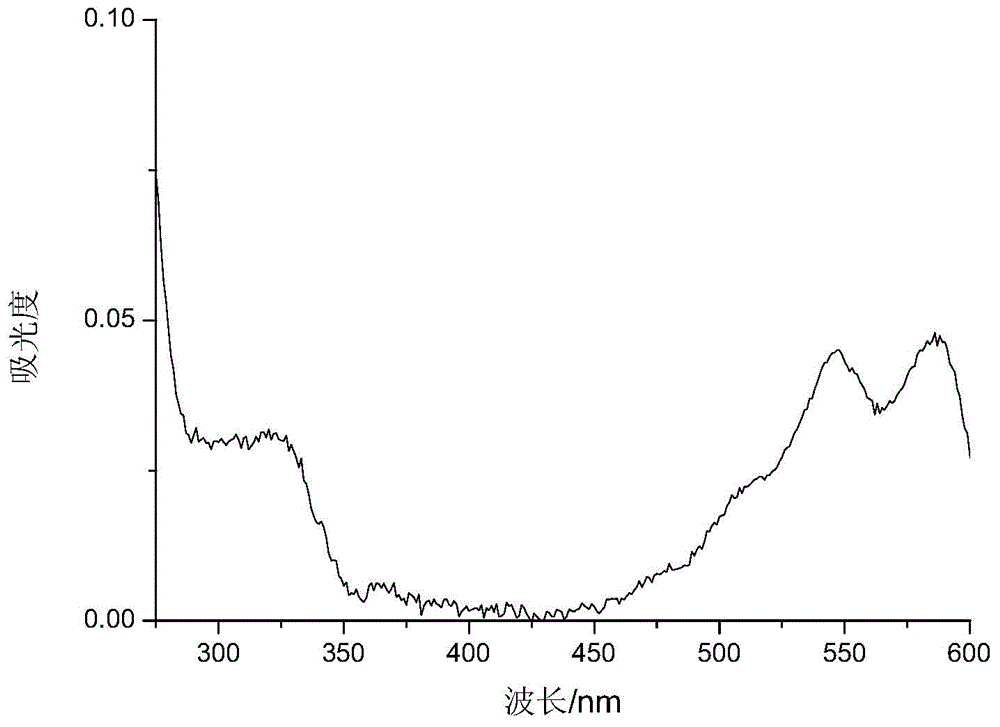
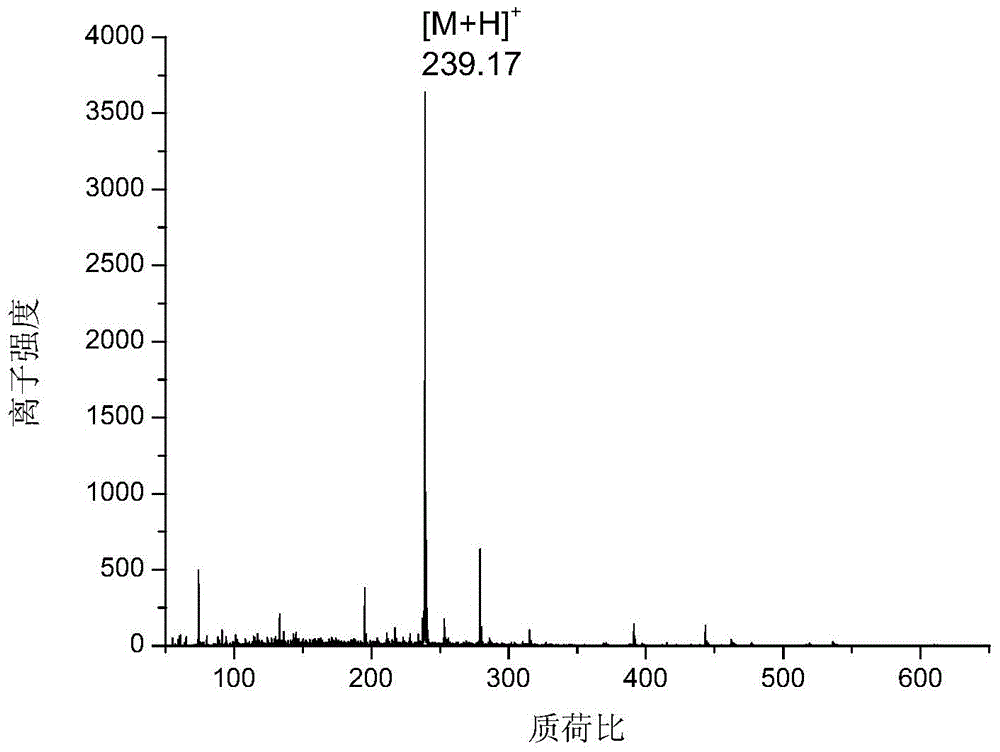
Application of anthraquinone or anthraquinone derivatives as matrix in matrix assisted ultraviolet-visible light laser desorption / ionization mass spectrometry
A technology of laser desorption ionization and anthraquinone derivatives, which is applied in the fields of environmental analysis, mass spectrometry detection, and metabolomics, and achieves the effects of good sample compatibility, reduced requirements, and wide practical significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
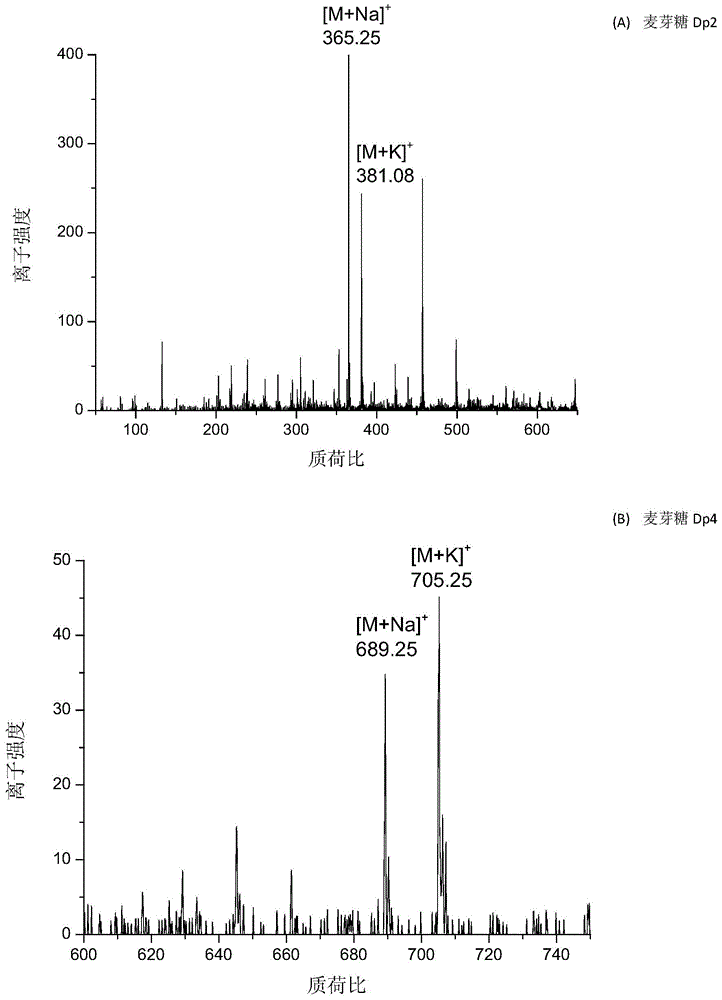
[0036] Embodiment 1, the analysis of oligosaccharides
[0037] Take various prepared sample solutions (maltose, maltotetraose and maltopentaose) and matrix solution and mix them uniformly at a volume ratio of 1:1 (wherein the molar ratio of matrix to maltose, maltotetraose and maltopentaose is 1000 :1, 1000:1 and 1000:1), then add 1 μL of the mixed sample on the ITO glass plate, dry it in the air and enter the mass spectrometry analysis. The wavelength of the light source is 532nm, the voltage of the ITO glass plate is 1.0kV, the temperature of the ion transfer tube is 275°C, the voltage of the lens is 40V, and the positive ion mode is used.
[0038] In the AP-Vis-MALDI ionization mode, the matrix can transfer protons to the analyte compounds (maltose, maltotetraose and maltopentaose), making the analyte compounds positively charged and detected by mass spectrometry.
[0039] The mass spectrometry figure that present embodiment obtains is as image 3 It can be seen from the ...
Embodiment 2
[0040] Embodiment 2, the analysis of polypeptide substance
[0041] Take various prepared sample solutions (tetrapeptide GPRP, hexapeptide GRGDTP and decapeptide TLSRRTRFHT) and matrix solution and mix them uniformly at a volume ratio of 1:1 (wherein, the moles of matrix and tetrapeptide GPRP, hexapeptide GRGDTP and decapeptide TLSRRTRFHT The ratios are 1000:1, 1000:1, and 1000:1), and then add 1 μL of the mixed sample to the ITO glass plate, dry it in the air, and enter the mass spectrometry analysis. The wavelength of the mass light source is 532nm, the voltage of the ITO glass plate is 1.0kV, the temperature of the ion transfer tube is 275°C, the voltage of the lens is 40V, and the positive ion mode is used.
[0042] In the AP-Vis-MALDI ionization mode, the matrix can transfer protons to the test compound (tetrapeptide GPRP, hexapeptide GRGDTP, decapeptide TLSRRTRFHT), so that the test compound is positively charged and detected by mass spectrometry.
[0043] The mass spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com