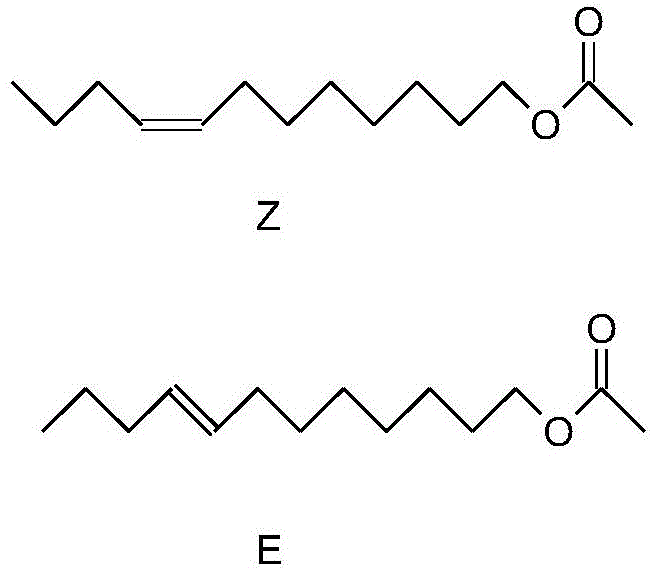
Synthetic method for (Z/E)-8-dodecylene-1-ol acetate compound
A technology of dodecene and alcohol acetate, applied in the field of pesticides, can solve problems such as unfavorable large-scale production, difficult post-processing, many side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
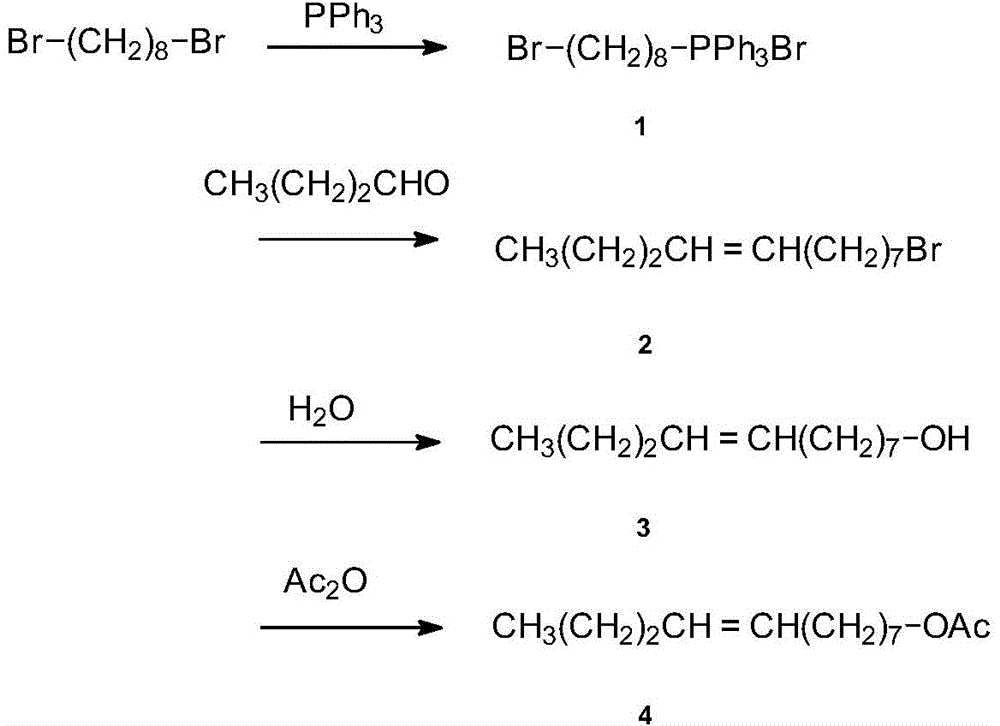
[0030] (1) Synthesis of ω-bromooctyltriphenylphosphonium bromide salt
[0031] Put 13.50 (50 mmol) of 1,8-dibromooctane and 13.10 (50 mmol) of triphenylphosphine into 150 ml of benzene, heat and reflux for 20 hours, after the reaction is completed, pour out the benzene layer, remove the remaining benzene, and obtain ω-bromo Substituent octyltriphenylphosphonium bromide salt 23.10g, yield 86.9%.
[0032] (2) Synthesis of ω-bromododecene
[0033] Under the protection of nitrogen, 22.30g (40mmol) ω-bromoquaternary phosphine salt was added to the mixed solvent (25ml tetrahydrofuran and 5ml diglyme), stirred and dissolved while maintaining the temperature at 20°C, slowly added 24ml2. 5mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stirred for 1h, then cooled to -15°C, added n-butyraldehyde, kept warm for 10h, followed by TLC detection, added ice water after the reaction of raw materials, adjusted with 10% hydrochloric acid Neutral pH, extracted with ether, comb...
Embodiment 2
[0039] (1) Synthesis of ω-bromooctyltriphenylphosphonium bromide salt
[0040] Put 13.50 (50 mmol) of 1,8-dibromooctane and 14.41 (55 mmol) of triphenylphosphine into 150 ml of benzene, heat and reflux for 18 hours, after the reaction is completed, pour out the benzene layer, remove the remaining benzene, and obtain ω-bromo Substituent octyltriphenylphosphonium bromide salt 23.10g, yield 87.50%.
[0041] (2) Synthesis of ω-bromododecene
[0042]Under the protection of nitrogen, add 26.60g (50mmol) ω-bromoquaternary phosphonium salt into the mixture of 25ml tetrahydrofuran and 5ml diethylene glycol dimethyl ether, stir to dissolve and maintain the temperature at 20°C-30°C, slowly add 25ml2 .5mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stirred for 1 hour, then cooled to -20°C, added n-butyraldehyde, kept for 13 hours, followed by TLC detection. Adjust the pH to be neutral, extract with ether, combine the organic phases, wash with saturated aqueous sodium ...
Embodiment 3
[0048] (1) Synthesis of ω-bromooctyltriphenylphosphonium bromide salt
[0049] Put 27.0g (100mmol) of 1,8-dibromooctane and 28.8g (110mmol) of triphenylphosphine into 300ml of benzene, heat and reflux for 24h, after the reaction is over, pour out the benzene layer, remove the remaining benzene, and obtain ω- Bromooctyltriphenylphosphonium bromide salt 48.10g, yield 90.50%.
[0050] (2) Synthesis of ω-bromododecene
[0051] Under the protection of nitrogen, add 31.92g (60mmol) ω-bromoquaternary phosphonium salt into the mixture of 30ml tetrahydrofuran and 6ml diethylene glycol dimethyl ether, stir to dissolve and maintain the temperature at 20°C-30°C, slowly add 30ml3 .0mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stirred and reacted for 1.5h, then cooled to -5°C, added dropwise a mixture of 4.32g (60mmol) n-butyraldehyde and 8ml tetrahydrofuran, kept for 10h, followed by TLC detection After the reaction of the raw materials, add ice water, adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com