Synthesis method for sex pheromone of ostriniafurnacalis
A synthetic method and a technology of corn borer sex, which is applied in the field of sex pheromones of the Asian corn borer, can solve the problems of high product impurities, many side reactions, and low solubility, and achieve the effects of high product purity, few side reactions, and economical process schemes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
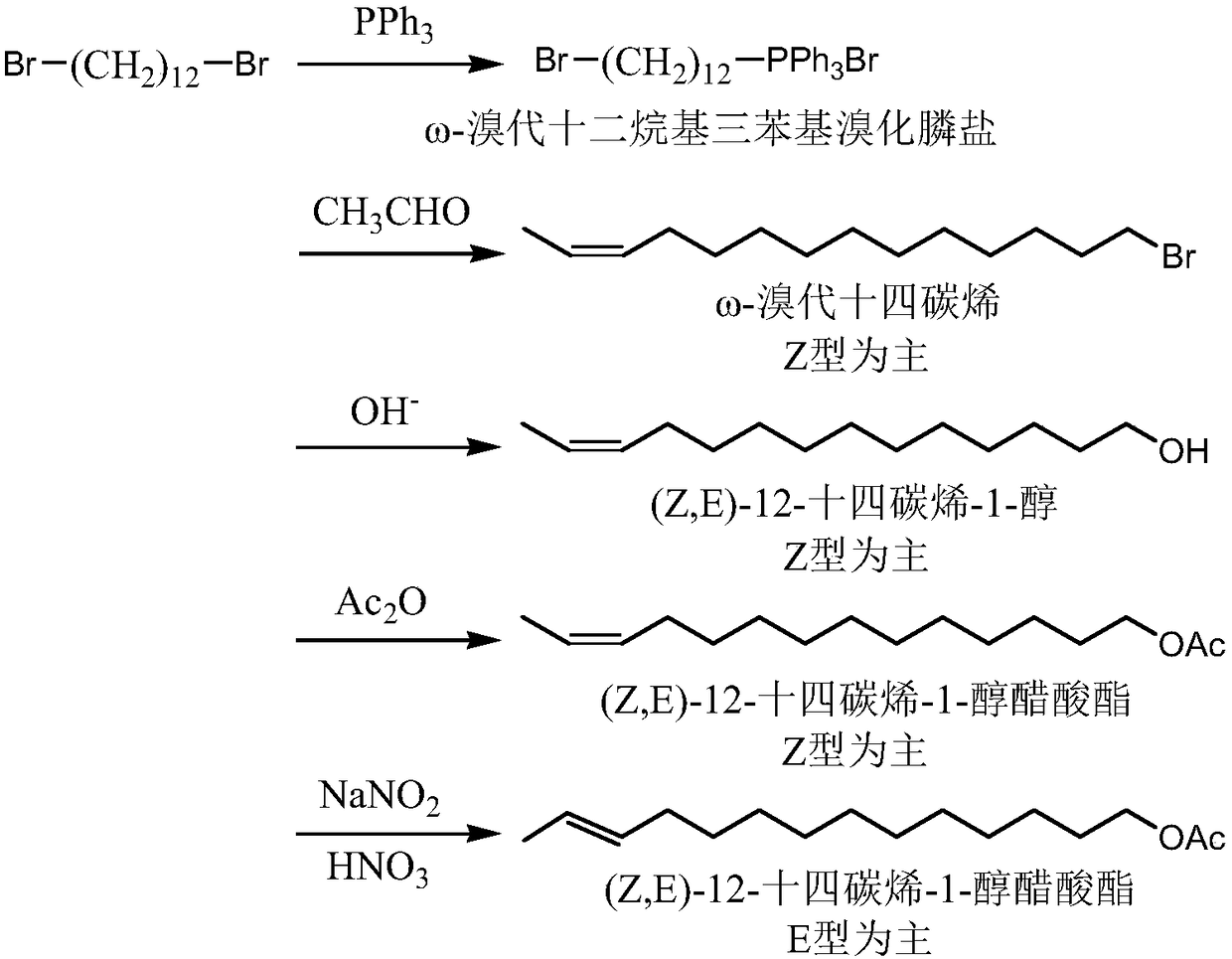
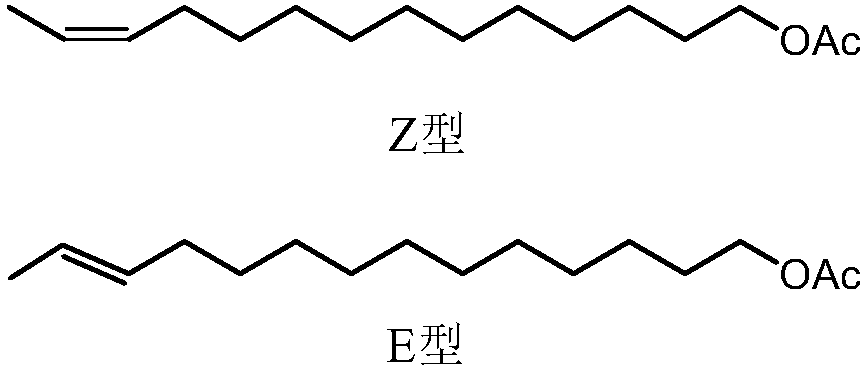
[0081] A method for synthesizing the sex pheromone (Z, E)-12-tetradecen-1-ol acetate compound of Ostrinia officinalis, comprising the steps of:
[0082] (1) Synthesis of ω-bromododecyltriphenylphosphonium bromide salt
[0083] Put 16.40g (50mmol) of 1,12-dibromododecane and 13.10g (50mmol) of triphenylphosphine into 50ml of benzene, heat and reflux for 20h, after the reaction is over, pour out the benzene layer, and remove the residual benzene to obtain ω-bromododecyltriphenylphosphonium bromide salt 25.52g, yield 86.5%.
[0084] (2) Synthesis of ω-tetradecene bromide
[0085]Under the protection of nitrogen, 23.60g (40mmol) ω-bromoquaternary phosphine salt obtained in step (1) was added to the mixed solvent (5ml tetrahydrofuran and 5ml diglyme), stirred and dissolved and maintained at 20°C , slowly add 19.2ml 2.5mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stir the reaction for 1h, then cool down to -15°C, add 1.76g (40mmol) acetaldehyde in 2mL tetrahyd...
Embodiment 2
[0093] A method for synthesizing the sex pheromone (Z, E)-12-tetradecen-1-ol acetate compound of Ostrinia officinalis, comprising the steps of:
[0094] (1) Synthesis of ω-bromododecyltriphenylphosphonium bromide salt
[0095] Put 16.40g (50mmol) of 1,12-dibromododecane and 14.41g (55mmol) of triphenylphosphine into 50ml of benzene, heat and reflux for 18h, after the reaction is complete, pour out the benzene layer, and remove the residual benzene to obtain ω-bromododecyltriphenylphosphonium bromide salt 25.87g, yield 87.7%.
[0096] (2) Synthesis of ω-tetradecene bromide
[0097] Under the protection of nitrogen, add 29.50g (50mmol) ω-bromoquaternary phosphonium salt into the mixture of 25ml tetrahydrofuran and 5ml diethylene glycol dimethyl ether, stir to dissolve and maintain the temperature at 20°C-30°C, slowly add 40ml 2.5mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stirred for 1h, then cooled to -20°C, added 2.42g (55mmol) acetaldehyde in 2mL tetra...
Embodiment 3
[0105] A method for synthesizing the sex pheromone (Z, E)-12-tetradecen-1-ol acetate compound of Ostrinia officinalis, comprising the steps of:
[0106] (1) Synthesis of ω-bromododecyltriphenylphosphonium bromide salt
[0107] Put 32.80g (100mmol) of 1,12-dibromododecane and 39.27g (150mmol) of triphenylphosphine into 100ml of benzene, heat and reflux for 24 hours, and when the reaction is complete, pour out the benzene layer and remove the remaining benzene to obtain 53.57 g of ω-bromododecyltriphenylphosphonium bromide salt, yield 90.8%.
[0108] (2) Synthesis of ω-tetradecene bromide
[0109] Under the protection of nitrogen, add 35.40g (60mmol) ω-bromoquaternary phosphonium salt into the mixture of 60ml tetrahydrofuran and 6ml diethylene glycol dimethyl ether, stir to dissolve and maintain the temperature at 20°C-30°C, slowly add 50ml 3.0mol / L tetrahydrofuran solution of dimethyl sulfoxide sodium salt, stirred and reacted for 1.5h, then cooled to -5°C, added dropwise a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com