Method for preparing S-2-(4-nitrobenzophenone) oxirane
A technology of nitrophenyl and ethylene oxide, which is applied in the field of biocatalytic asymmetric preparation of pharmaceutical chiral intermediates, can solve the problems of difficult industrial production and environmental protection, low product yield, and high cost of preparation columns, achieving reduction Effects of acid-base consumption, high catalytic efficiency, strong enzyme stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
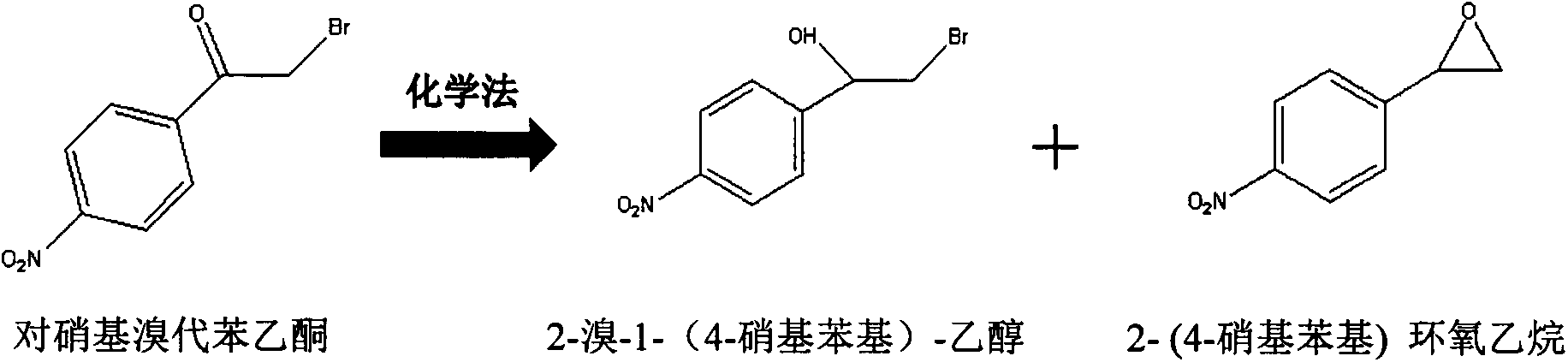
[0021] Embodiment 1 The chemical synthesis of racemate 2-bromo-1-(4-nitrophenyl)-ethanol and 2-(4-nitrophenyl)oxirane mixture
[0022] Dissolve 5g of racemate p-nitrobromoacetophenone and 1g of sodium borohydride in 50mL of methanol, stir with a stirring bar, react in an ice bath environment for 3h, add 50mL of water, and at the same time add 300mL of ethyl acetate Shake the ester in a separatory funnel, keep the organic phase, and wash the organic phase with MgSO 4 Dry, and spin dry with a rotary evaporator to obtain racemate 2-bromo-1-(4-nitrophenyl)-ethanol: racemate 2-(4-nitrophenyl)oxirane (7: 3) The solid of the mixture is 4 g.
Embodiment 2
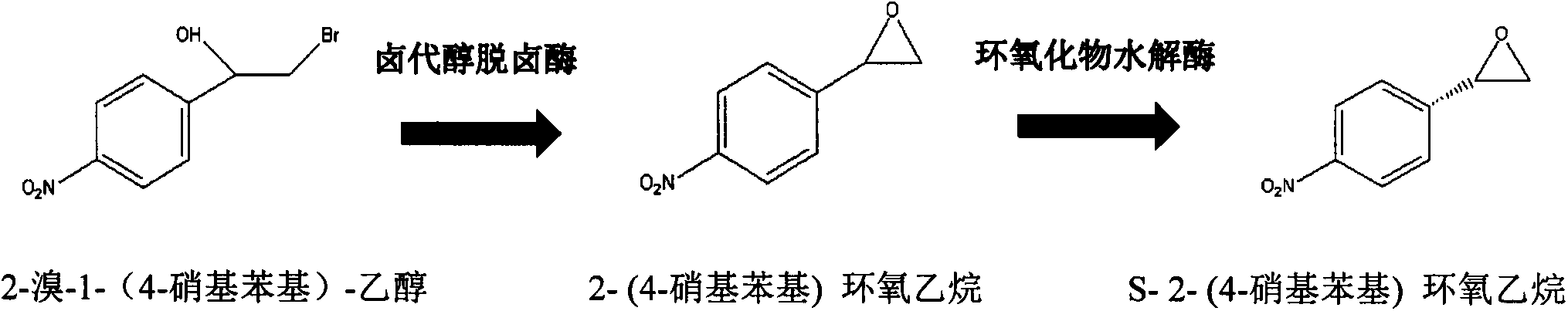
[0023] Enzyme-catalyzed generation of embodiment 2S-2-(4-nitrophenyl)oxirane
[0024] Centrifuge 100mL of fermentation broth to obtain 1.5g of halohydrin dehalogenase wet body bacteria and add it to 1000mL of transformation liquid, which contains 4g / L of the above mixture, 100mL of N,N-dimethylformamide, and 900mL of phosphate buffer , pH8.0, react for 1h. Then add 100mL of fermentation broth, centrifuge to obtain 1.5g of epoxide hydrolase wet bacteria, react for 30min, and determine the reaction end point by GC gas phase detection. After the reaction, the concentration of S-2-(4-nitrophenyl)oxirane in the conversion solution was 1.3 g / L.
Embodiment 3
[0025] The separation and purification of embodiment 3S-2-(4-nitrophenyl) oxirane
[0026] The transformation solution was centrifuged at 4000 rpm for 15 minutes to remove the bacteria, and 100 mL of ethyl acetate was added to the supernatant to shake well, and the organic phase was retained, and 1.2 g of solid was obtained by rotary evaporation. The solid substance was passed through a silica gel column, and the target substance was eluted with a mixture of ethyl acetate:petroleum ether (1:7). The eluate was passed through a rotary evaporator to obtain 1.0 g of S-2-(4-nitrophenyl)oxirane solid with high purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com