Method for degrading polyethylene glycol terephthalate (PET)
A technology of polyethylene terephthalate and ethanol, applied in the field of polyethylene terephthalate and degradation of polyethylene terephthalate, can solve the problems of transparency and sanitation, and regenerate Low physical properties of materials, environmental pollution and other problems, to achieve high yield, fast reaction speed, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
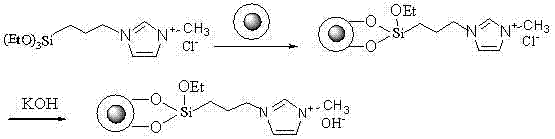
Image
Examples
Embodiment 1
[0024] FeCl 2 4H 2 O (1.85 mmol), FeCl 3 ·6H 2 O (3.7mmol) was dissolved in 30ml deionized water at room temperature and under the protection of argon, the resulting solution was added to 25% ammonia solution (10mL) with vigorous mechanical stirring (300rpm). A black precipitate was formed instantaneously. In order to obtain tiny and uniform γ-Fe 2 o 3 Granules, NH 4 The dropwise reaction rate of OH should be accurately controlled, after 15min, Ca(NO 3 ) 2 4H 2 O (33.7 mmol, 0.5 M) and (NH 4 ) 2 HPO 4 (20 mmol, 3.0 M) solution (pH=11) in 100ml, added dropwise to the obtained precipitate within 30 minutes of stirring, and mechanically stirred for 30 minutes. The resulting emulsion mixture was heated to 90 o C. After 2 h, the mixture was cooled to room temperature and aged overnight. The resulting dark brown precipitate was filtered, washed repeatedly with deionized water until neutral, and dried in vacuum air at room temperature. The synthesized sample is at 30...
Embodiment 2
[0026] FeCl 2 4H2 O (1.85 mmol), FeCl 3 ·6H 2 O (3.7mmol) was dissolved in 30ml deionized water at room temperature and under the protection of argon, the resulting solution was added to 25% ammonia solution (10mL) with vigorous mechanical stirring (300rpm). A black precipitate was formed instantaneously. In order to obtain tiny and uniform γ-Fe 2 o 3 Granules, NH 4 The dropwise reaction rate of OH should be accurately controlled, after 15min, Ca(NO 3 ) 2 4H 2 O (33.7 mmol, 0.5 M) and (NH 4 ) 2 HPO 4 (20 mmol, 3.0 M) solution (pH = 11) in 100ml, added dropwise to the obtained precipitate within 30 minutes of stirring, and mechanically stirred for 30 minutes. The resulting emulsion mixture was heated to 100 o C. After 2 h, the mixture was cooled to room temperature and aged overnight. The resulting dark brown precipitate was filtered, washed repeatedly with deionized water until neutral, and dried in vacuum air at room temperature. The synthesized sample is at 30...
Embodiment 3
[0028] FeCl 2 4H 2 O (1.85 mmol), FeCl 3 ·6H 2 O (3.7mmol) was dissolved in 30ml deionized water at room temperature and under the protection of argon, the resulting solution was added to 25% ammonia solution (10mL) with vigorous mechanical stirring (300rpm). A black precipitate was formed instantaneously. In order to obtain tiny and uniform γ-Fe 2 o 3 Granules, NH 4 The dropwise reaction rate of OH should be accurately controlled, after 15min, Ca(NO 3 ) 2 4H 2 O (33.7 mmol, 0.5 M) and (NH 4 ) 2 HPO 4 (20 mmol, 3.0 M) solution (pH = 11) in 100ml, added dropwise to the obtained precipitate within 30 minutes of stirring, and mechanically stirred for 30 minutes. Heat the resulting emulsion mixture to 120 o C. After 2 hours, the mixture was cooled to room temperature and aged overnight. The resulting dark brown precipitate was filtered, washed repeatedly with deionized water until neutral, and dried in vacuum air at room temperature. The synthesized sample is at 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com