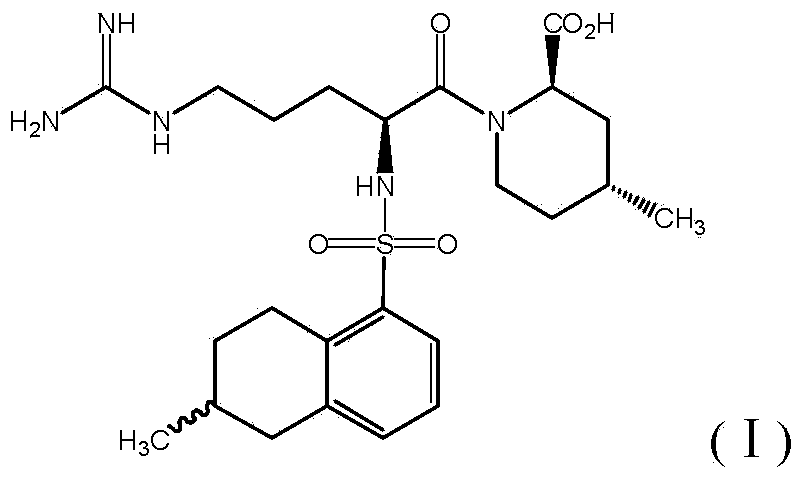
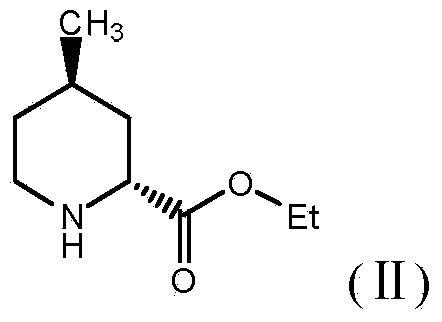
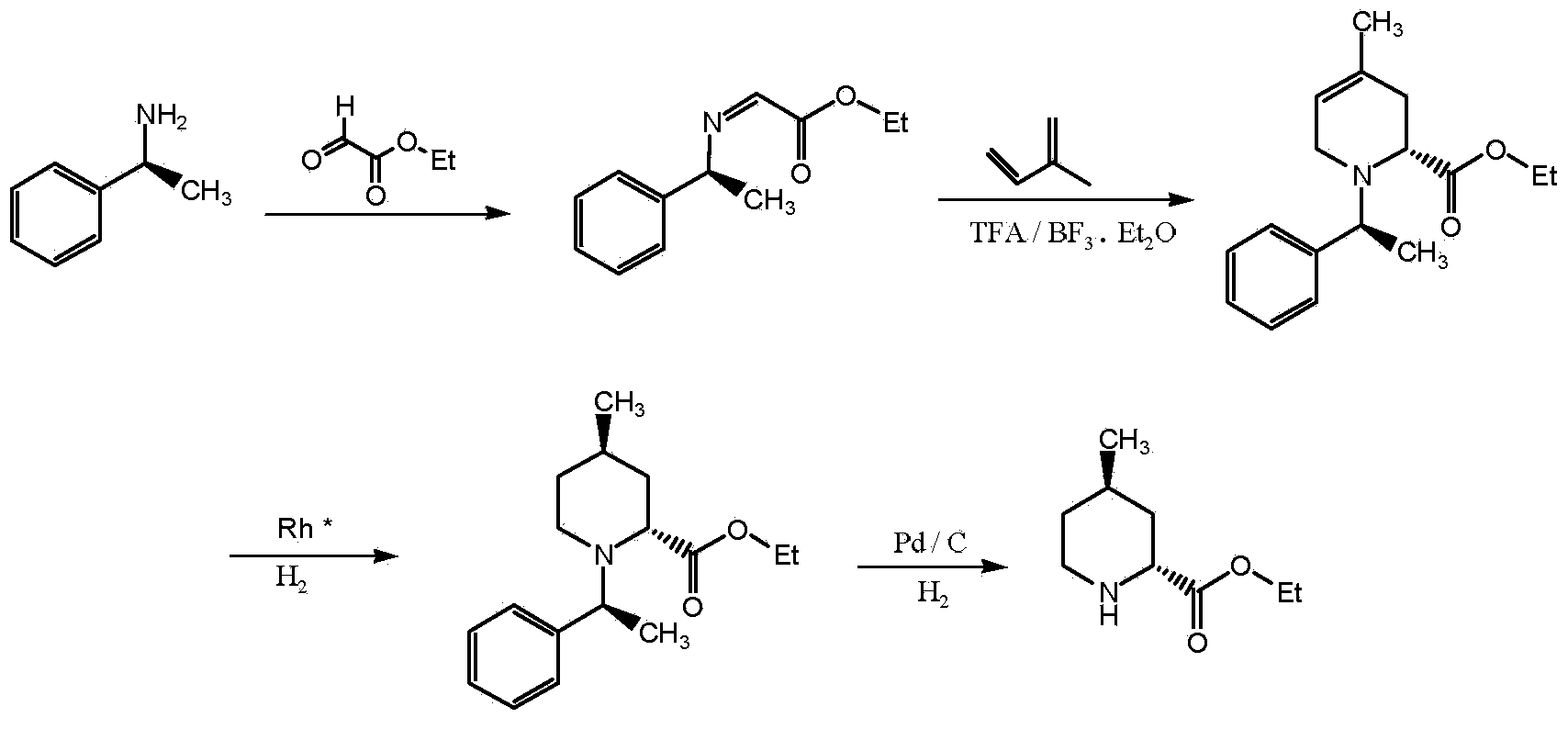
Preparation method of (2R, 4R)-4-pipecolines-2-ethyl formate compound
A technology of ethyl formate and methylpiperidine, which is applied in the field of preparation of ethyl-4-methylpiperidine-2-carboxylate, can solve the problems of low yield, long synthetic route, poor reaction selectivity, etc. Easy to obtain, high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of ethyl (2R, 4R)-4-methyl-1-((S)-1-phenethyl)tetrahydropyridine-2-carboxylate
[0046] Combine (2R)-4-methyl-1-((S)-1-phenethyl)-1,2,3,6-tetrahydropyridine-2-carboxylic acid ethyl ester (328g, 1.2mol) and ethanol ( 2500mL) into a 5L autoclave, add rhodium-alumina catalyst (10% rhodium loading, 60g), and pass H 2 , React at 35℃, 1MPa for 12h, filter, and recover the catalyst. The reaction solution was concentrated under reduced pressure, ethyl acetate (1000 mL) was added, washed with saturated brine (250 mL x 2), dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure to obtain a colorless and transparent liquid product (310 g).
[0047] Liquid chromatography determination: (2R, 4R)-4-methyl-1-((S)-1-phenethyl)-2-piperidine ethyl ester content is 73.2%.
[0048] 2. Preparation of (2R, 4R) ethyl-4-methylpiperidine-2-carboxylate
[0049] Put the crude product (200g) and ethanol (1000mL) obtained in the above steps...
Embodiment 2
[0055] 1. Preparation of ethyl (2R, 4R)-4-methyl-1-((S)-1-phenethyl)tetrahydropyridine-2-carboxylate
[0056] Combine (2R)-4-methyl-1-((S)-1-phenethyl)-1,2,3,6-tetrahydropyridine-2-carboxylic acid ethyl ester (328.1g, 1.2mol) and ethanol (2500mL) into a 5L autoclave, add rhodium-carbon catalyst (5% rhodium loading, 50g), pass H 2 , React at 40℃, 1Mpa for 12h. Filter and recover the catalyst. The reaction solution was concentrated under reduced pressure, ethyl acetate (1000mL) was added, washed with saturated brine (250mL x 2), dried over anhydrous sodium sulfate and filtered. The filtrate was decolorized by adding silica gel and filtered. The filtrate was concentrated under reduced pressure to obtain colorless and transparent Liquid product (315g).
[0057] Determination by liquid chromatography: (2R, 4R)-4-methyl-1-((S)-1-phenethyl)-2-piperidine ethyl ester content is 68.5%.
[0058] 2. Preparation of (2R, 4R) ethyl-4-methylpiperidine-2-carboxylate
[0059] Put the crude product (1...
Embodiment 3
[0065] 1. Preparation of ethyl (2R, 4R)-4-methyl-1-((S)-1-phenethyl)tetrahydropyridine-2-carboxylate
[0066] Combine (2R)-4-methyl-1-((S)-1-phenethyl)-1,2,3,6-tetrahydropyridine-2-carboxylic acid ethyl ester (273g, 1.0mol) and ethanol ( 2000mL) into a 5L autoclave, add rhodium-alumina catalyst (5% rhodium loading, 75g), pass H 2 , React at 25℃, 0.5Mpa for 12h. Filter and recover the catalyst. The reaction solution was concentrated under reduced pressure, ethyl acetate (1000mL) was added, washed with saturated brine (250mL x 2), dried over anhydrous sodium sulfate and filtered. The filtrate was decolorized by adding silica gel and filtered. The filtrate was concentrated under reduced pressure to obtain colorless and transparent Liquid product (250g).
[0067] Liquid chromatography determination: (2R, 4R)-4-methyl-1-((S)-1-phenethyl)-2-piperidine ethyl ester content is 62.3%.
[0068] 2. Preparation of (2R, 4R) ethyl-4-methylpiperidine-2-carboxylate
[0069] Put the crude product (25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com