Synthetic method of oxadiazon
A synthetic method, the technology of oxadiazone, which is applied in the field of pesticide synthesis technology, can solve the problems of non-industrialization process, polluting the environment, restricting the popularization and use of oxadiazone products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: synthetic oxadiazone
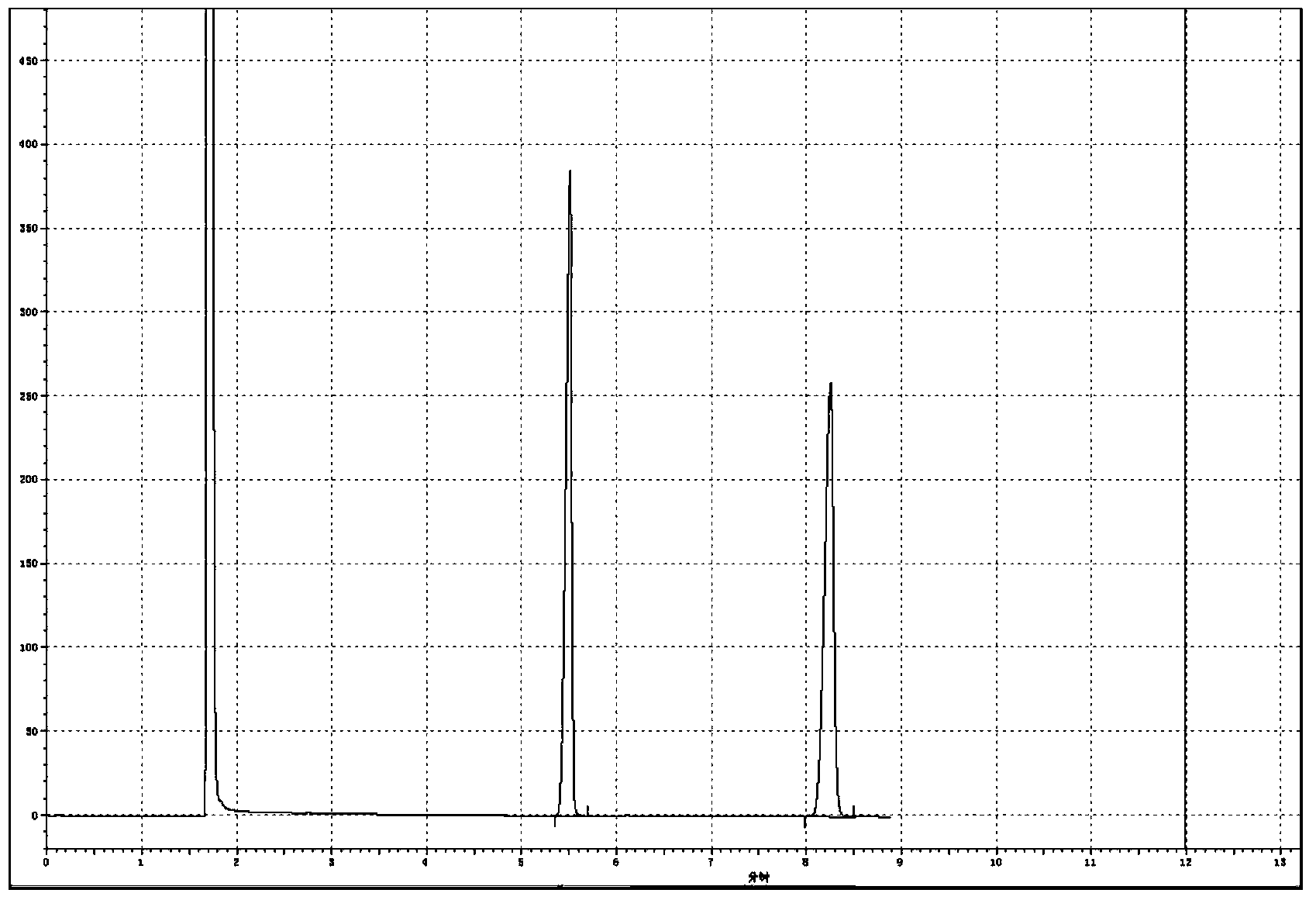
[0029] Add 2.0Kmol of 2,4-dichloro-5-isopropoxyphenylhydrazide (100%), 5.0Kmol of dimethyl carbonate, catalyst ( 30% sodium methoxide) 0.036Kmol, after the addition, stir, heat up to reflux, after reflux for 4 hours, take a sample to detect that the residual hydrazide is 0.2%, which is regarded as the end of the reaction, cool to -5°C, crystallize, centrifuge, and dry to obtain Oxadiazone 670.9kg, 0.45%, was regarded as the end point of the reaction, cooled to -6°C, crystallized, centrifuged, and dried to obtain oxadiazone 663.8kg, which was detected by gas chromatography at the same time. Chloromethane is dissolved, and tetradecane is used as an internal standard, and a certain chromatographic peak retention time in the resulting product solution ( figure 2 ) and the retention time of oxadiazone chromatographic peak in oxadiazone standard solution ( figure 1 ), the relative difference is within 1.5%, and the chromatographic peak ...
Embodiment 2
[0031] Embodiment 2: synthetic oxadiazone
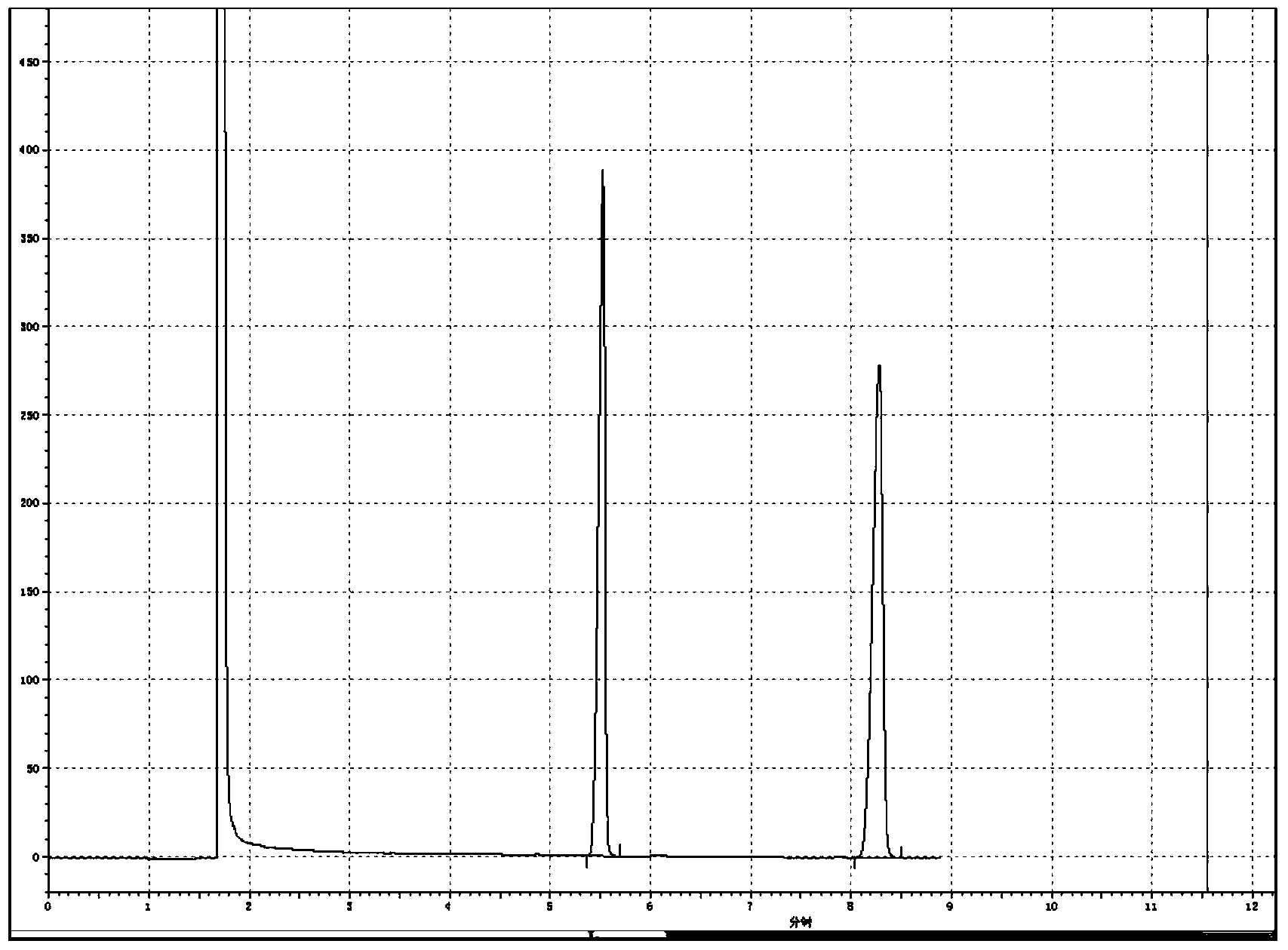
[0032] Add 2,4-dichloro-5-isopropoxyphenylhydrazide 2.0Kmol (100%), dimethyl carbonate 4.5Kmol, catalyst ( 30% sodium methoxide) 0.05Kmol, after the addition, stir, heat up to reflux, after 4h of reflux reaction, take a sample to detect that the hydrazide residue is 0.22%, which is regarded as the end of the reaction, cool to -5°C, crystallize, centrifuge, and dry to obtain Oxadiazone 667.3kg, 0.45%, was regarded as the end point of the reaction, cooled to -6°C, crystallized, centrifuged, and dried to obtain oxadiazone 663.8kg, which was detected by gas chromatography at the same time. Chloromethane is dissolved, and tetradecane is used as an internal standard, and a certain chromatographic peak retention time in the resulting product solution ( image 3 ) and the retention time of oxadiazone chromatographic peak in oxadiazone standard solution ( figure 1 ), the relative difference was within 1.5%, and the chromatographic peak was ...
Embodiment 3
[0034] Embodiment 3: synthetic oxadiazone
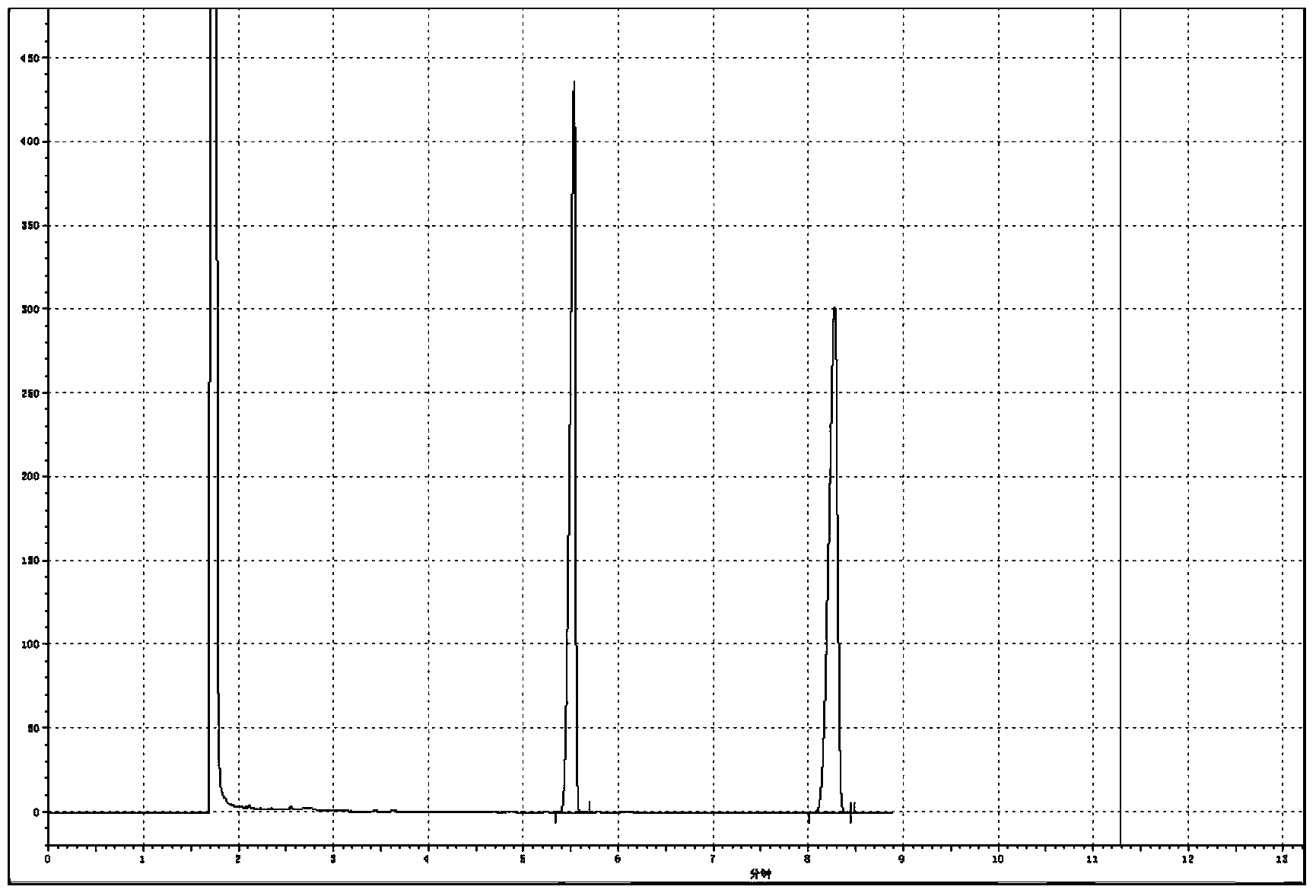
[0035] Add 2.0Kmol of 2,4-dichloro-5-isopropoxyphenylhydrazide (100%), 4.0Kmol of dimethyl carbonate, catalyst ( 30% sodium methoxide) 0.02Kmol, after the addition, stir, heat up to reflux, after 6h of reflux reaction, take a sample to detect 0.42% hydrazide residue, which is regarded as the end of the reaction, cool to -3°C, crystallize, centrifuge, and dry to obtain Oxadiazone 640.7kg, 0.45%, was regarded as the end point of the reaction, cooled to -6°C, crystallized, centrifuged, and dried to obtain oxadiazone 663.8kg, which was detected by gas chromatography at the same time. Chloromethane is dissolved, and tetradecane is used as an internal standard, and a certain chromatographic peak retention time in the resulting product solution ( Figure 4 ) and the retention time ( figure 1 ), the relative difference was within 1.5%, and the chromatographic peak was identified as oxadiazone. The internal standard method detected oxadiazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com