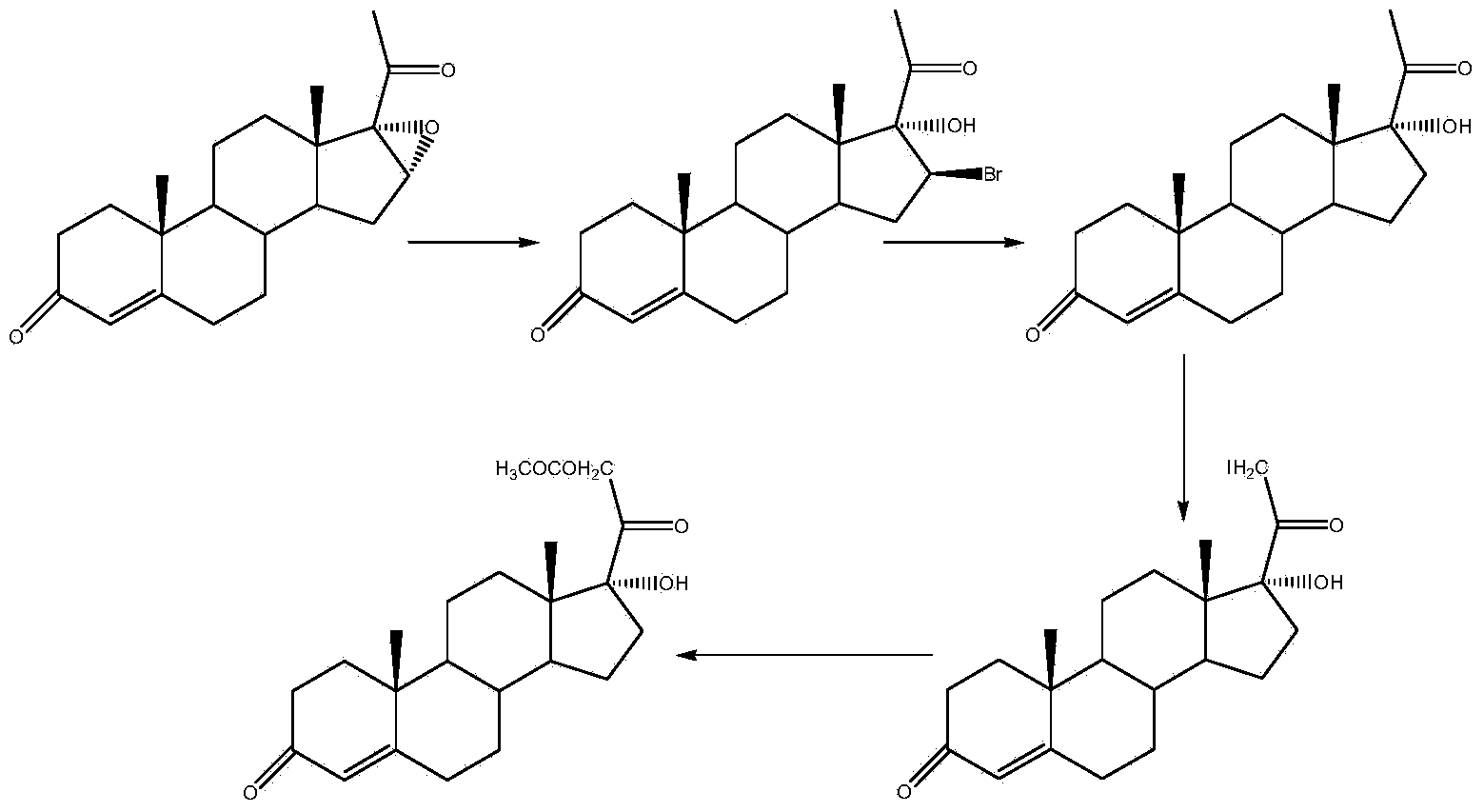
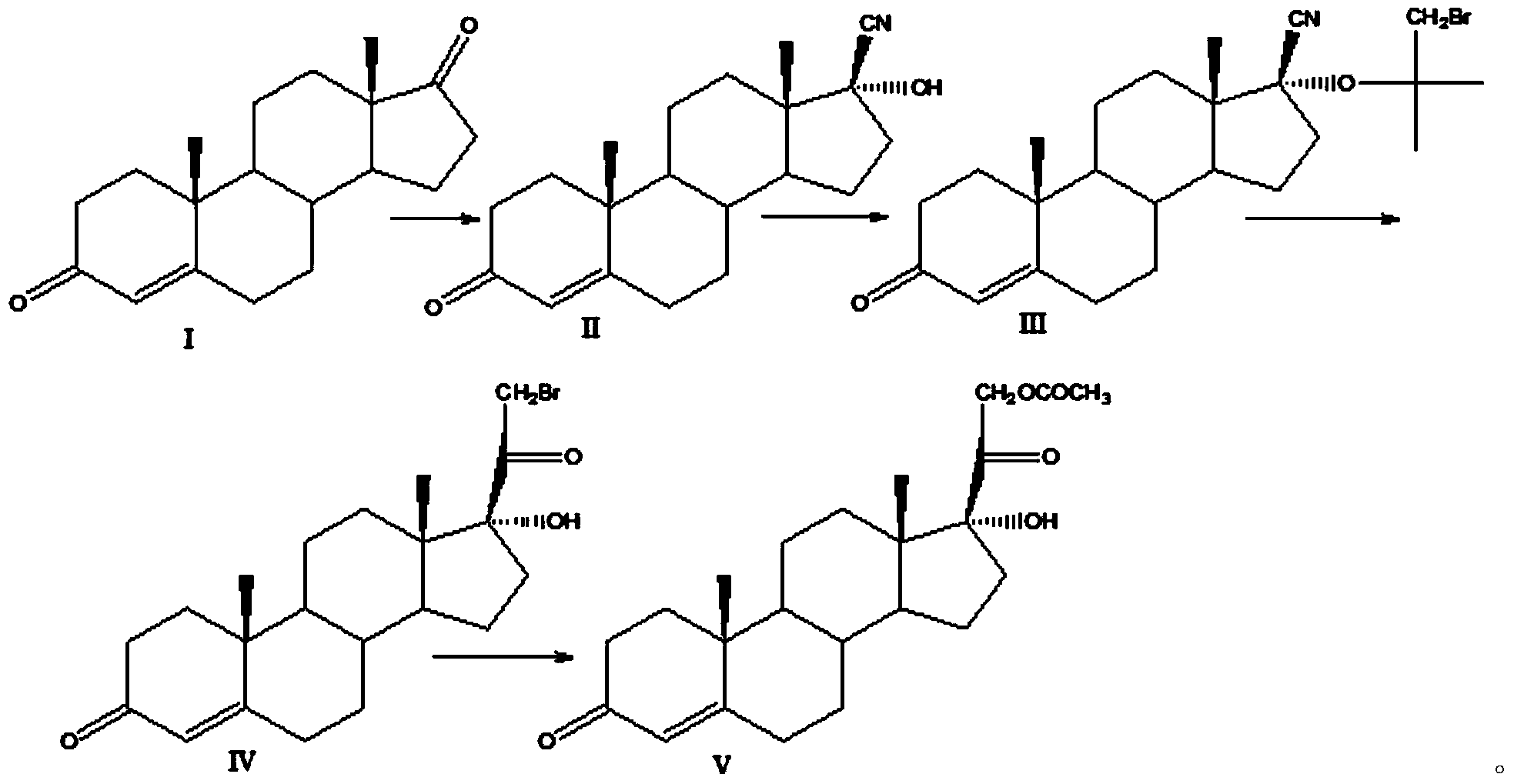
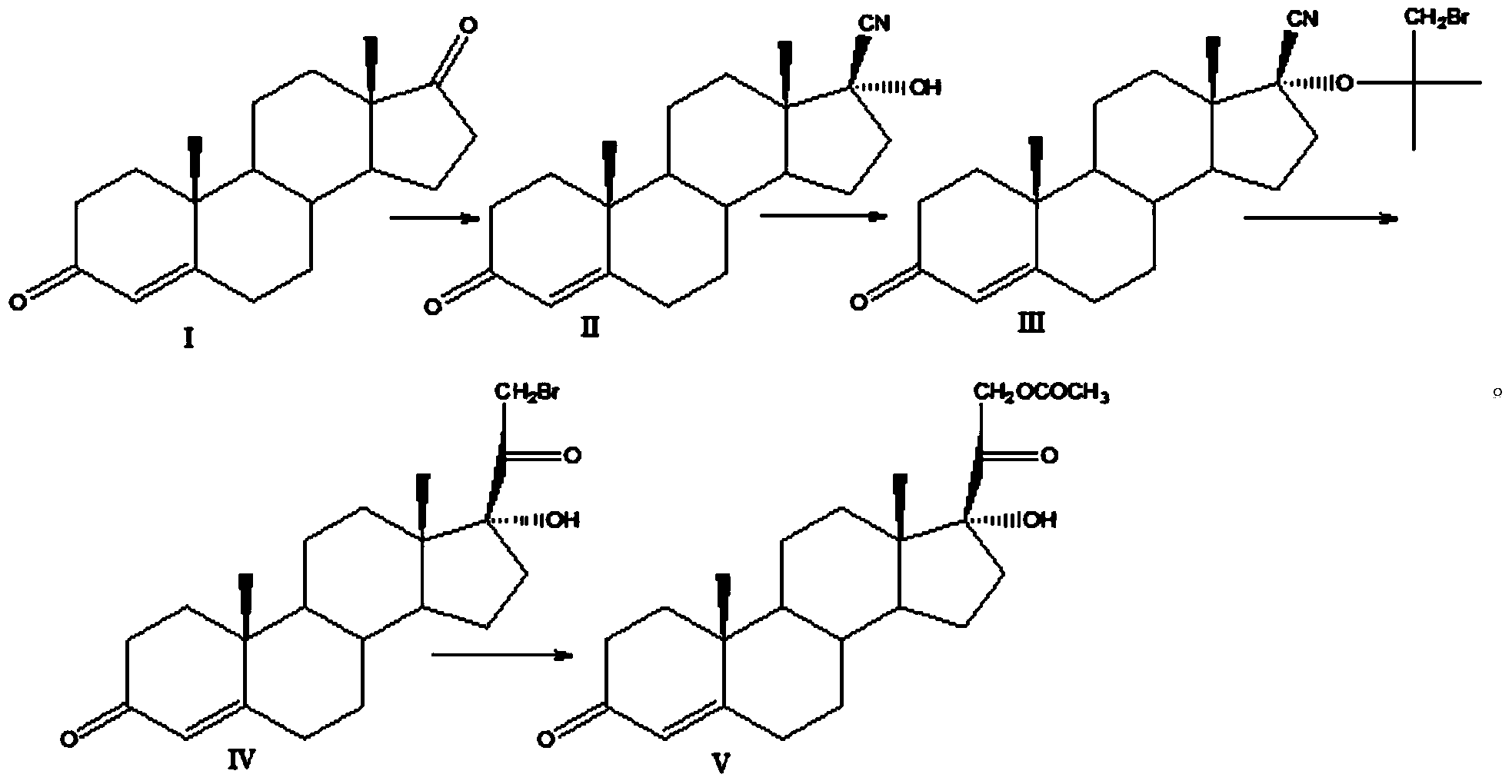
Preparation method for hydrocortisone intermediate
A technology of hydrocortisone and intermediates, applied in the field of chemical synthesis of steroid hormone drugs, can solve the problems of high production requirements, high process cost, long synthesis steps, etc., to reduce the dependence of high-end equipment and achieve high yield , the effect of short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Cyano substitution reaction
[0021] At room temperature, add 200ml of methanol, 100ml of acetone cyanohydrin, and 200.0g of compound I to a clean and dry 2000ml four-necked round-bottomed flask equipped with a thermometer, reflux condenser, and mechanical stirring. After stirring evenly, add 200ml of Potassium carbonate aqueous solution, the temperature of the system was controlled at 40-50° C. for 30 hours, and TLC detected that the raw materials were no longer reduced. The reaction system was added dropwise to 4000ml of water and stirred for 2 hours. Suction filtration, washing the filter cake with water to neutrality, and drying at 50°C to obtain 210 g of compound II with a yield of 105% and a HPLC purity of 97.8%.
[0022] Siloxyl Protection Reaction
[0023] At room temperature, add 250ml of chloroform, 100.0g of compound II, and 52g of imidazole to a clean and dry 2000ml four-necked round-bottom flask equipped with a thermometer, reflux condenser, and mechanica...
Embodiment 2
[0029] Cyano substitution reaction
[0030] At room temperature, add 300ml of acetone, 120g of acetone cyanohydrin, and 200.0g of compound I to a clean and dry 2000ml four-necked round-bottom flask equipped with a thermometer, reflux condenser, and mechanical stirring. After stirring evenly, add 150ml of sodium carbonate solution, the temperature of the system was controlled at 40-50° C. for 20 hours, and TLC detected that the raw materials were no longer reduced. 1000 ml of water was added dropwise to the reaction system, and stirred for 2 hours. Suction filtration, washing the filter cake with water to neutrality, and drying at 50°C to obtain 208g of compound II, yield: 104%, HPLC purity 97.5%.
[0031] Siloxyl Protection Reaction
[0032] At room temperature, add 600ml of toluene, 100g of compound II, and 54g of triethylamine to a clean and dry 2000ml four-necked round-bottomed flask equipped with a thermometer, reflux condenser, and mechanical stirring in sequence. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com