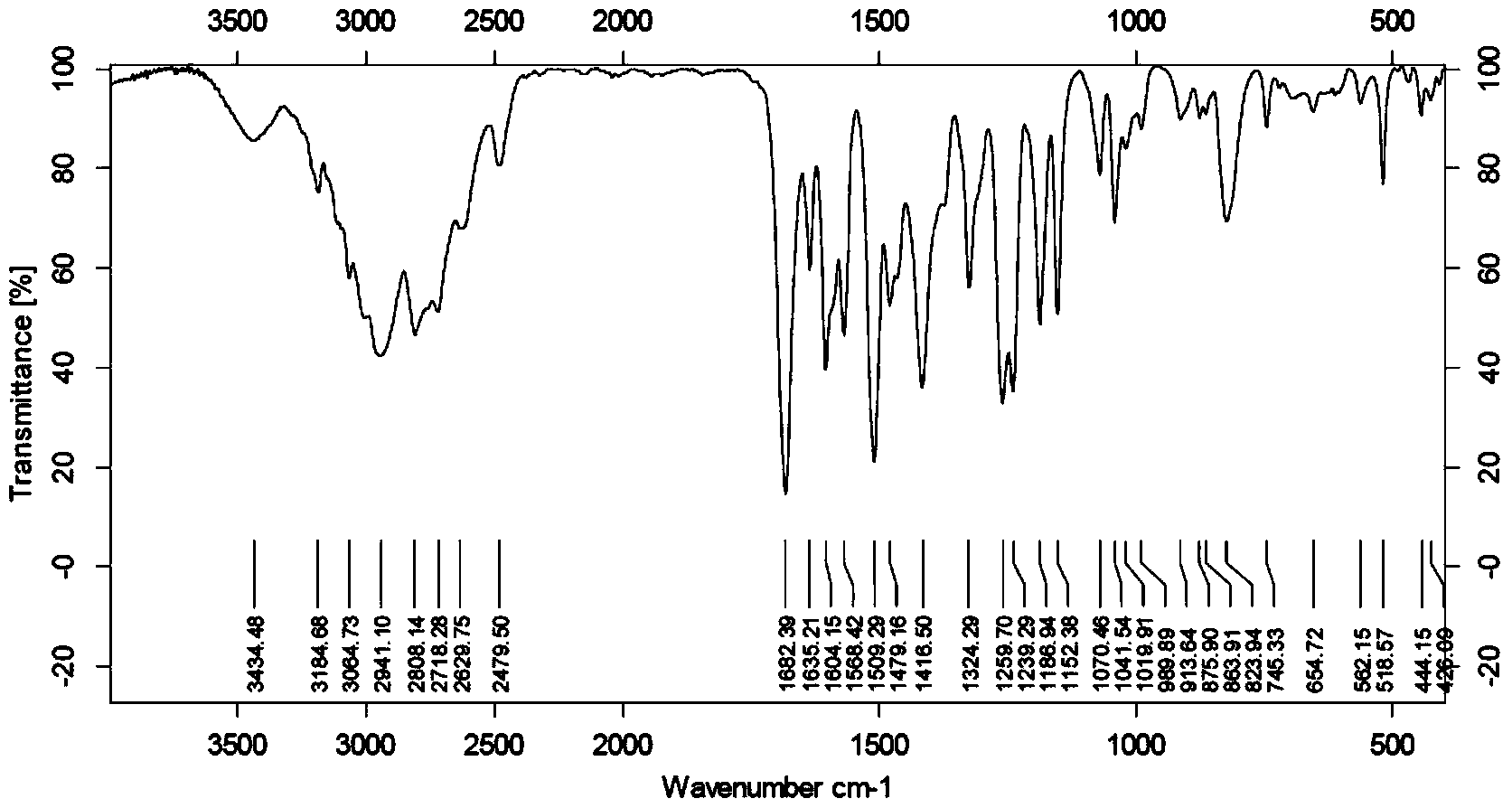
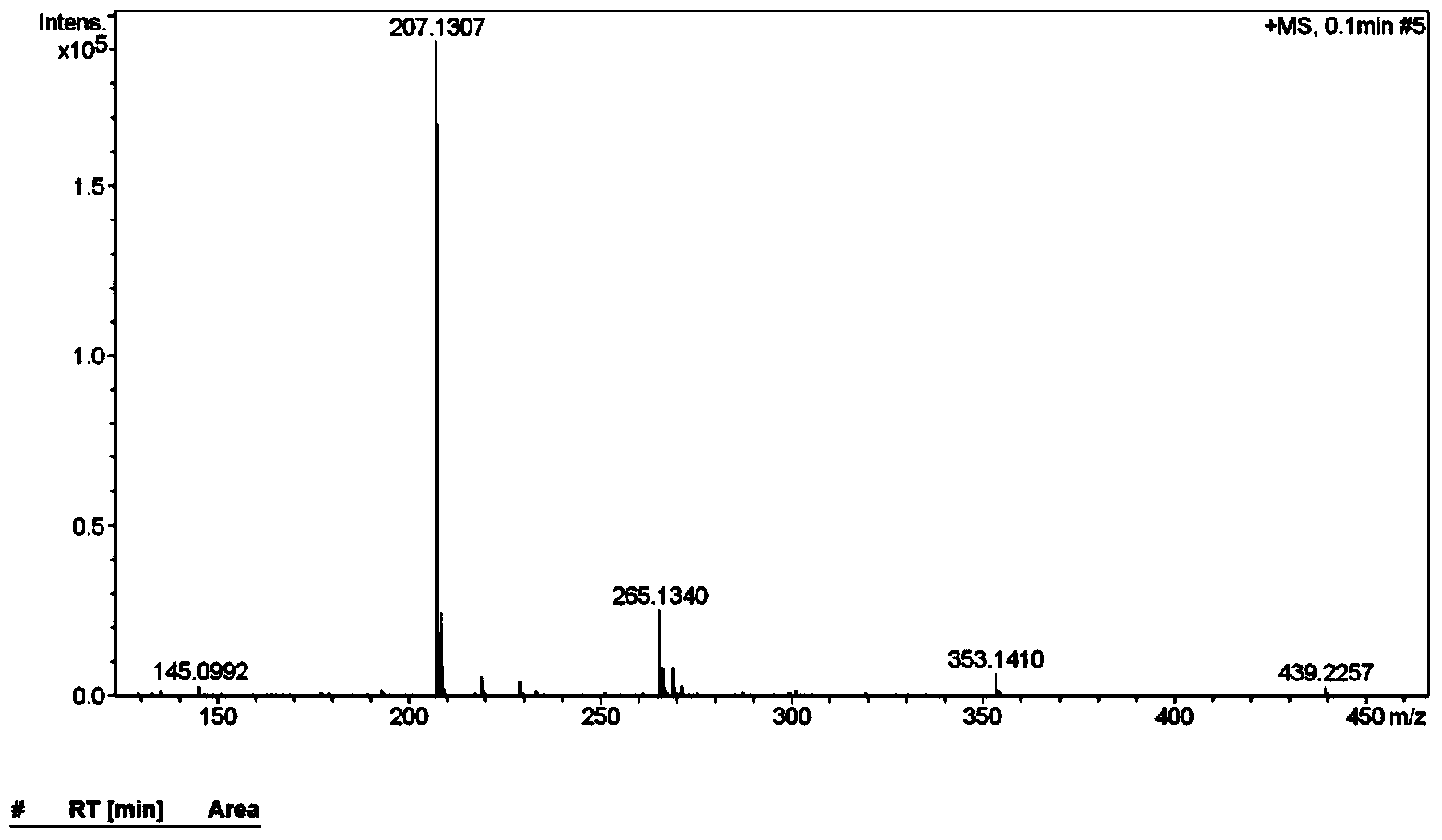
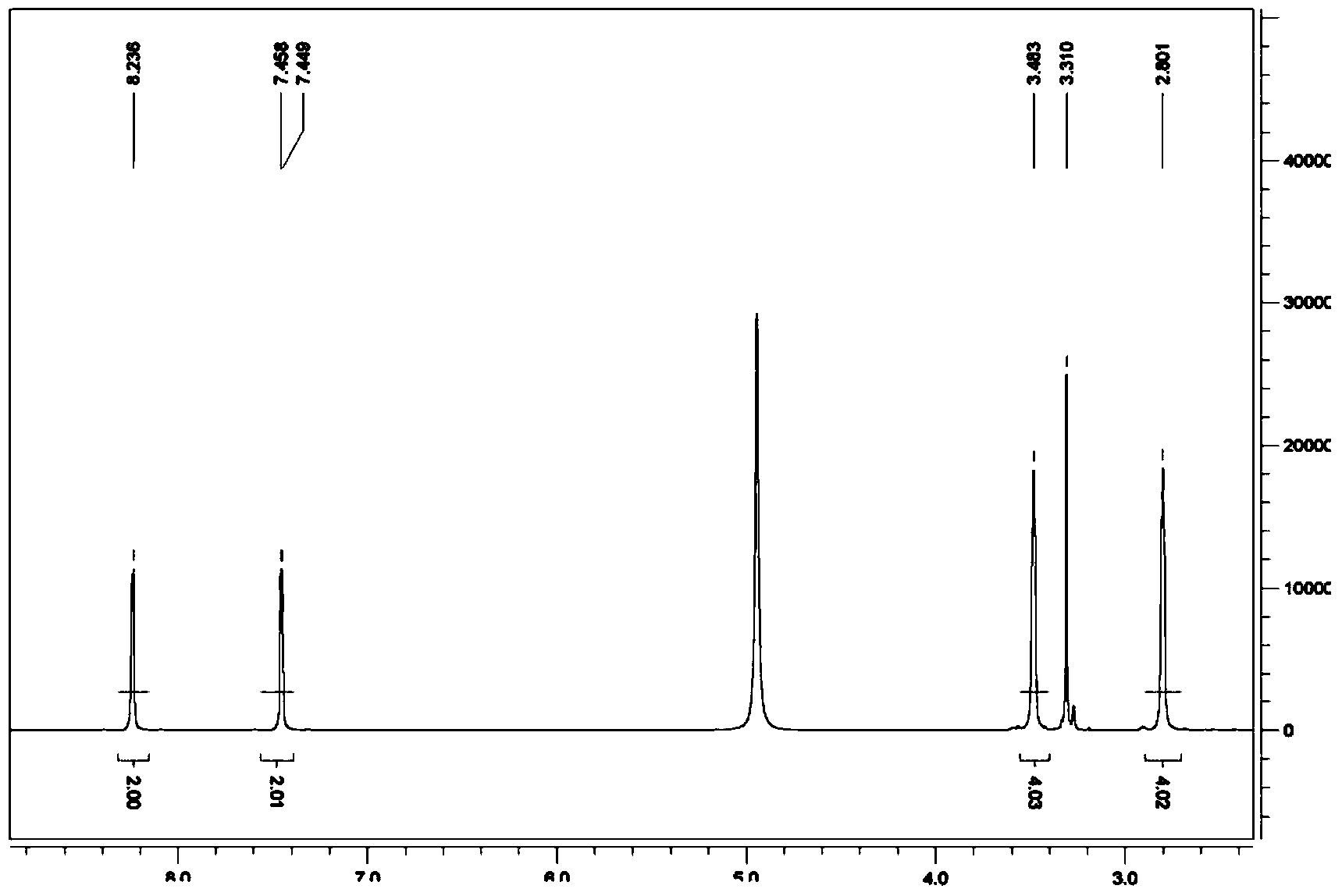
Use of N-(pyridine-4-yl) piperazine-1-formamide
A formamide and formamide compound technology, applied in the field of medicine, can solve problems such as fast metabolism, achieve low toxic and side effects, and inhibit inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 N-(pyridine-4-)piperazine-1-carboxamide
[0058] step one:
[0059] Disperse 4-aminopyridine (3.76g, 39.95mmol) in 50mL of dichloromethane, add triethylamine (4.85g, 47.94mmol), mix well, add ethyl chloroformate (4.34g, 39.95mmol) dropwise under ice cooling mmol), followed by overnight reaction at room temperature. Evaporate the solvent under reduced pressure, adjust the solution to PH=5-6 with 5% dilute hydrochloric acid solution, extract with ethyl acetate (50mL×3), combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate under reduced pressure. 5.80 g of white solid was obtained, yield 87.3%. .
[0060] Step two:
[0061] Dissolve the white solid (3.00g, 18.06mmol) and piperazine (3.03g, 35.16mmol) in 30mL of 1,4-dioxane, and add 1mL of N-methylpyrrolidine as a catalyst, then reflux for 24h, reduce The solvent was evaporated under pressure, and column chromatography [V (ethyl acetate): V (methanol)...
Embodiment 2
[0072] Example 2 Test of Rho Kinase Inhibitor Activity
[0073] Microglia are immune cells in the central nervous system. They can not only protect neurons by phagocytizing pathogens and harmful particles in brain tissue, but also activate reactive microglia under the action of inflammatory molecules. , which secretes inflammatory cytokines and has toxic effects on neurons, is an important target for the treatment of neuroinflammation and neurodegenerative diseases. BV2 cells are the most commonly used microglia for in vitro studies. Treat with N-(pyridine-4-)piperazine-1-carboxamide and PBS respectively (PBS is the abbreviation of phosphate buffer solution, generally active biological agents should be diluted with it, because it has salt balance, can Adjusted appropriate pH buffering effect) was used as a control test, and its activity was detected with a Rho kinase activity kit after 24 hours of action.
[0074] Figure 5 Is N-(pyridine-4-)piperazine-1-carboxamide Rho kin...
Embodiment 3
[0075] Example 3 Test of Cell Regeneration and Synapse Formation Ability
[0076] Image 6 is the effect of N-(pyridine-4-)piperazine-1-carboxamide on synapse formation (24h) in primary neurons and BV-2 microglia. We used mouse primary neurons and BV-2 microglial cell line to observe the effect of War-5 on cell synapses. The results showed that War-5 was able to promote synapse formation to neurons compared to the PBS control. Using the BV-2 microglial cell line, we also obtained similar results.
[0077] Figure 7 It is N-(pyridine-4-)piperazine-1-carboxamide treatment of synapse formation in BV-2 microglial cells at different times (5d). We further observed the effect of War-5 treatment at different times on cell synapse formation. The results show that War-5 can promote synapse regeneration, and the drug effect lasts longer.
[0078] Figure 8 is N-(pyridine-4-)piperazine-1-carboxamide for quantification of mean synapse length in microglia. The results show that N-(p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com