Amine compound, preparation method thereof and application of amine compound in preparation of anti-influenza virus medicine
An amine compound and anti-influenza virus technology, applied in the field of medicine and biology, can solve problems such as obvious side effects, gastrointestinal adverse reactions, drug cross-resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: General synthetic steps for compounds 1 and 2:
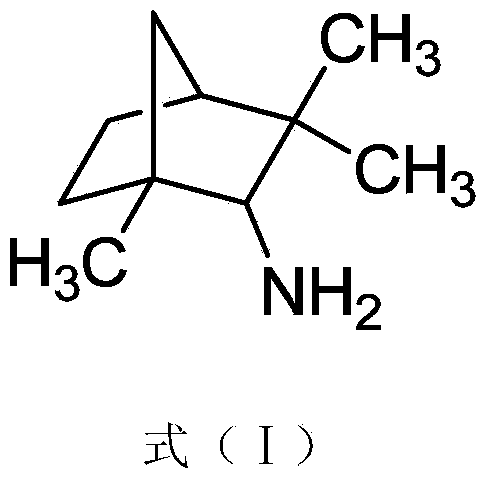
[0022] Compound 1:
[0023]
[0024] A synthetic route: This compound is a known compound and commercial compound, which can be purchased from related reagent companies, so no synthetic route is required. Purchase URL: http: / / www.sigmaaldrich.com / catalog / product / aldrich / 179604?lang=zh®ion=CN. CAS#: 7242-92-4.
[0025] B Chemical Characterization: Not required.
[0026] Compound 2:
[0027]
[0028] A synthetic route:
[0029]
[0030] B Raw material and abbreviation explanation
[0031]
[0032]
[0033] C chemical characterization
[0034] 1. Intermediate products
[0035]
[0036] Add hydroxylamine hydrochloride (5g, 65.7mmol), pyridine 20ml in a 100ml eggplant-shaped flask, stir in an oil bath at 80°C for 5min until hydroxylamine hydrochloride is completely dissolved and clear. Add fenchone (2g, 13.14mmol), heat to reflux, and stir overnight. After overnight, the reaction was st...
Embodiment 2
[0040] Embodiment 2: Influenza virus suppression experiment
[0041]Experimental materials: MDCK dog kidney cells; H1N1 influenza virus A / PR / 8 / 34 (M2 wild type); culture medium DMEM+10%FBS, maintenance solution DMEM+2%FBS (DMEM, Hyclone; FBS, Gibco); Hanks buffer; CCK-8 (Donjindo, Japan); Amantadine (Sigma Aldrich).
[0042] experimental method
[0043] 1. Determination of virus virulence (TCID50 of virus virulence is 10 -5.5 )
[0044] MDCK cells were divided into 5×10 4 Inoculate each well in a 96-well plate to form a confluent monolayer of cells, add 100 μl of 10-fold serially diluted virus solution of 6 concentrations (the virus stock solution is derived from chicken embryo proliferation, diluted in DMEM) after adsorption for 1-2 hours, pour out the virus , rinsed twice with Hanks solution, added maintenance solution, observed cytopathic changes (CPE) every day with an inverted microscope, measured hemagglutination titer, observed cell survival status and virus amplifi...
Embodiment 3
[0049] Example 3: Detection of Compounds for Inhibition of H1N1-M2 Ion Channel Activity
[0050] 1. Construction of HEK293T-rex cell line stably expressing wild-type H1N1-M2 ion channel (Sun J, Li C, Xu W, Li Z, Liu J, Chen L. Chin J Biotech.2008,24(11):1902- 1906.).
[0051] 2. Patch clamp technique to detect the blocking effect of compounds on H1N1-M2 ion channels
[0052] HEK293T-rex cells expressing H1N1-M2 ion channels were induced by 1 μg / ml tetracycline for 24-48 hours, and the current changes before and after drug addition were recorded by patch clamp at room temperature (Hu W, Zeng S, Li C, Jie Y, Li Z, Chen L. J Med Chem. 2010, 53(9): 3831-3834.).
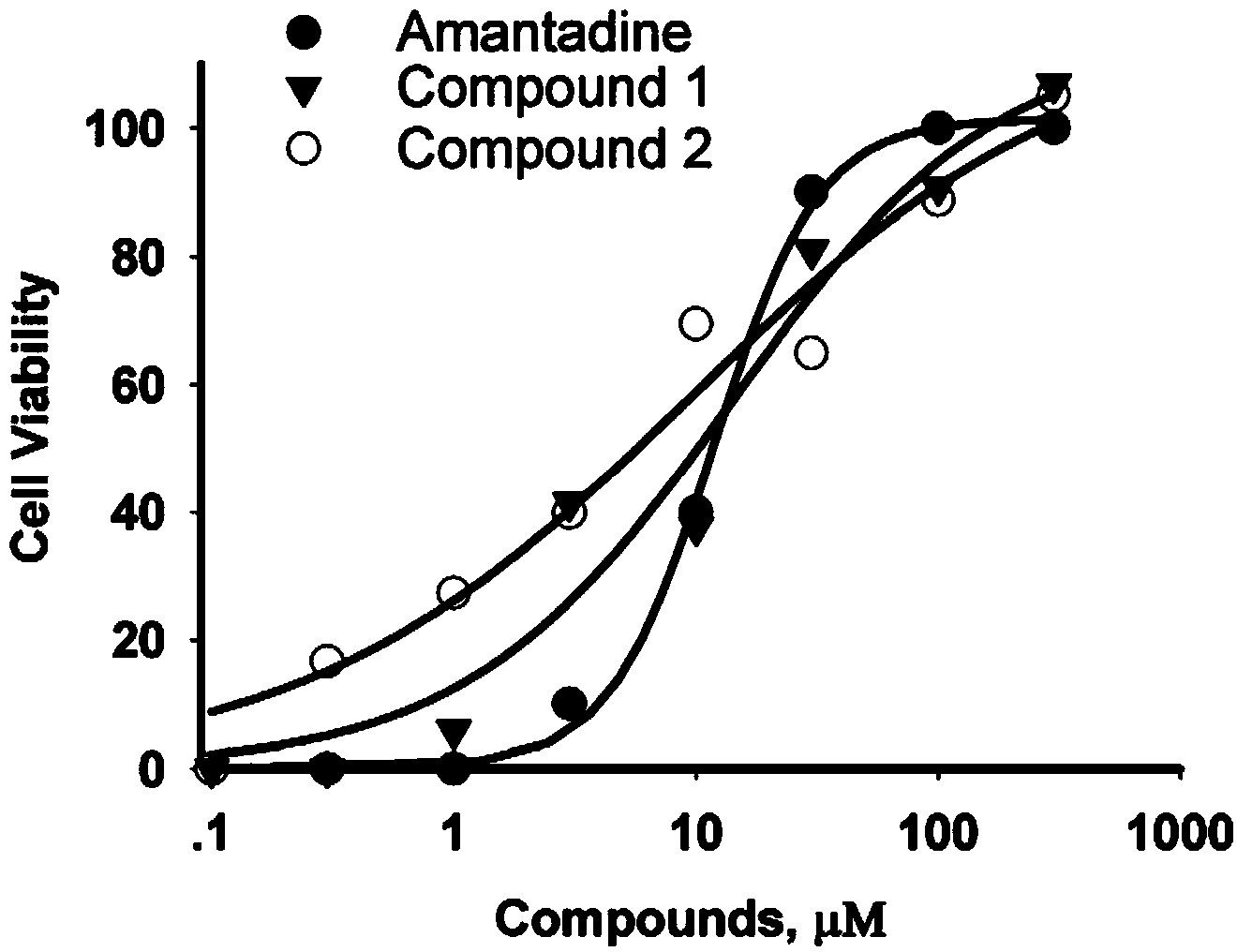
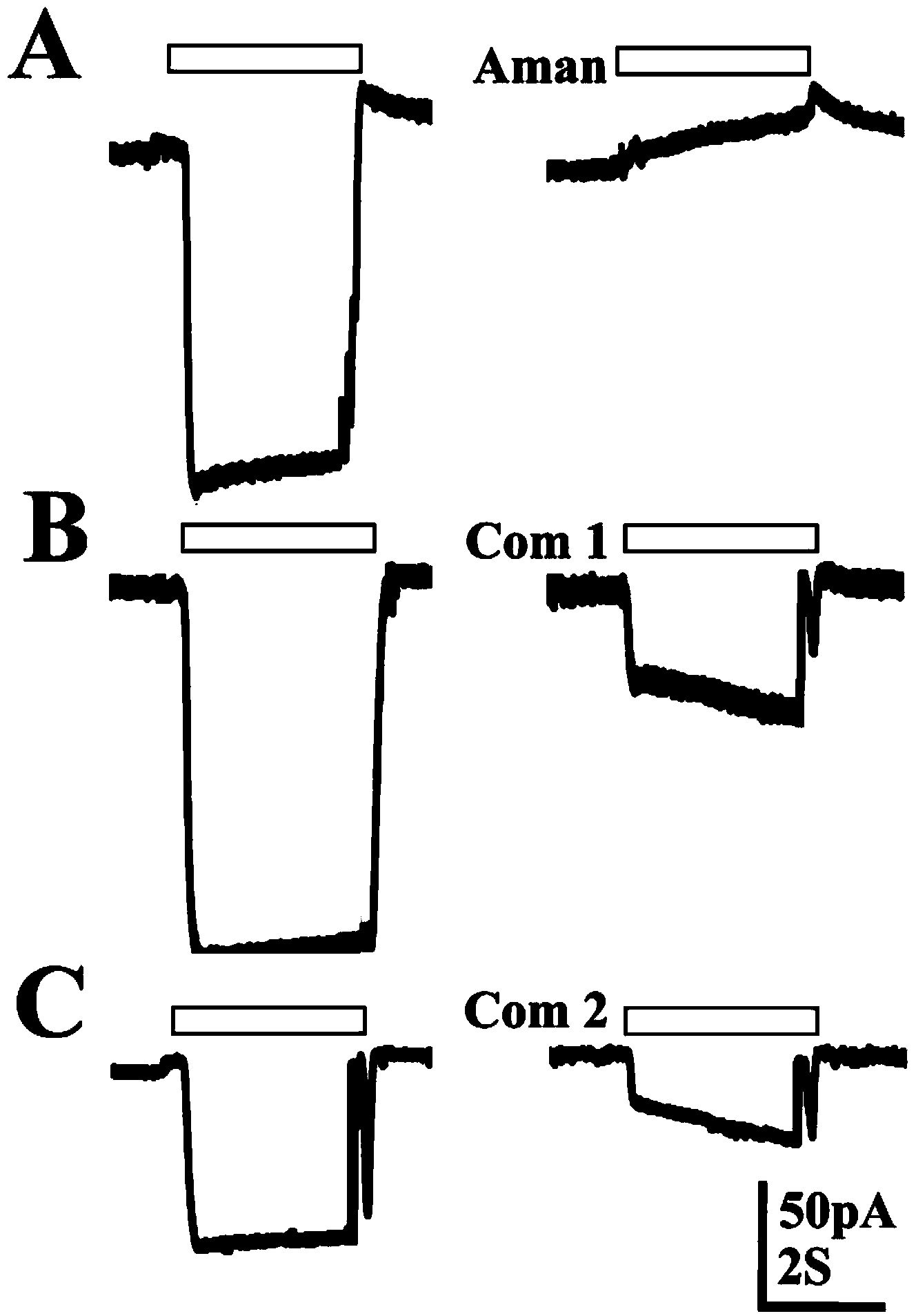
[0053] Results: 100 μmol / L compound 1 blocked the H1N1-M2 ion channel current by 76.8%, 100 μmol / L compound 2 blocked the current by 70.7%, and 100 μmol / L amantadine (Amantadine) could completely block the current ( figure 2 ), it can be seen that compound 1 and compound 2 have M2 ion channel inhibitory activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com