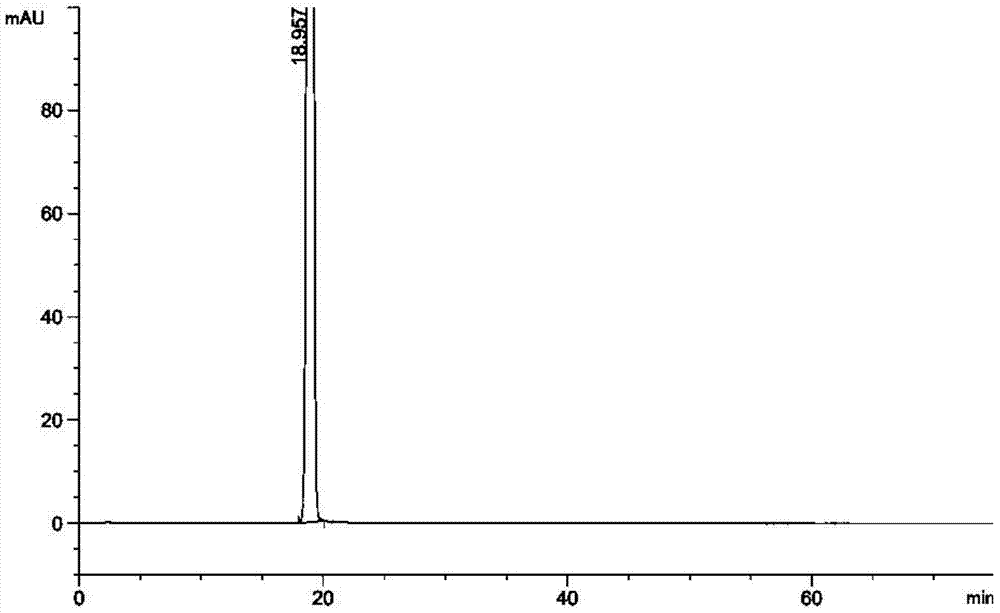
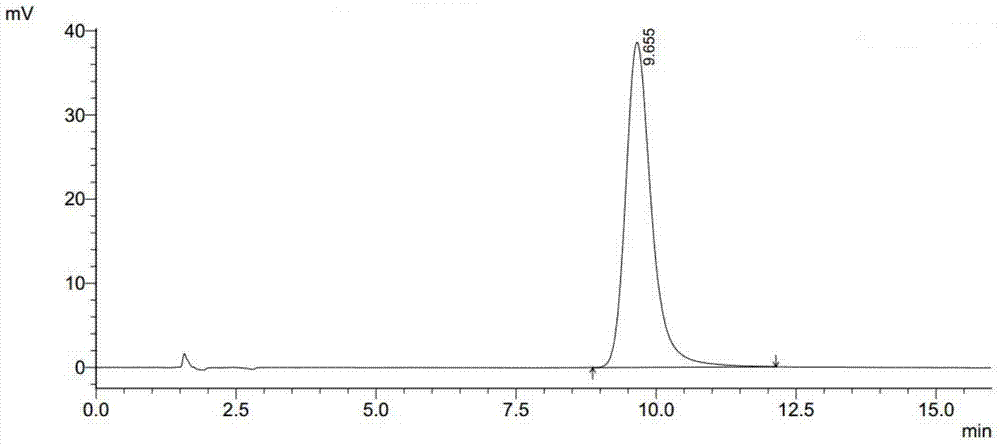
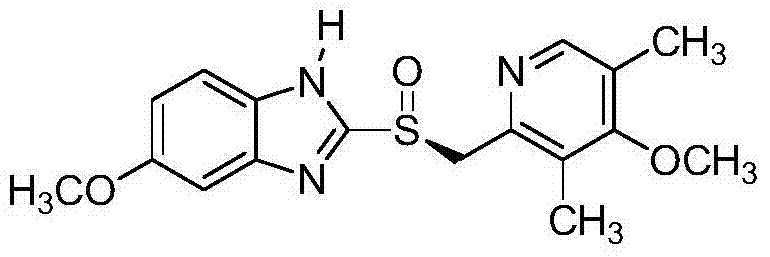
Preparation method of esomeprazole
A technology for esomeprazole sodium and omeprazole sodium, which is applied in the field of preparation of esomeprazole sodium, can solve the problems of high solvent toxicity, cumbersome operation, unsatisfactory purity and the like, and achieves controllable cost and easy operation. Simplicity, the effect of improving chemical and optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 The present embodiment is the preparation embodiment of esomeprazole sodium
[0053] Step (1) preparation of intermediate 1
[0054] Add 150ml of absolute ethanol and 3.0g of sodium hydroxide into a 250ml three-necked flask, heat and stir at 25-30°C for 2 hours, and then add 20.0g of omeprazole. Add 4.1 g of disodium hydrogen phosphate, continue stirring for 1 hour, transfer the reaction solution into a 250 ml single-necked bottle, evaporate to dryness in a water bath at 35-40 °C under reduced pressure, add 200 ml of acetone to the residue, stir and analyze at 10-15 °C crystal. After filtration, the filter cake was dried under reduced pressure at 35-40°C to obtain intermediate 1 with a yield of 98%, a purity of 99.914%, and a pH value of 10.8. The molar ratio of disodium hydrogen phosphate to omeprazole is 1:2.
[0055] Step (2) preparation of intermediate 2
[0056] Intermediate 1 prepared in step (1) was added with 12 g of titanium isopropoxide and 8....
Embodiment 2
[0066] Embodiment 2 The present embodiment is the embodiment of preparing esomeprazole sodium
[0067] Step (1) preparation of intermediate 1
[0068] Add 150ml of absolute ethanol and 3.0g of sodium hydroxide into a 250ml three-necked flask, heat and stir at 25-30°C for 3 hours, and then add 20g of omeprazole. Add 8.2 g of disodium hydrogen phosphate, continue to stir for 1.5 hours, transfer the reaction solution into a 250 ml single-necked bottle, evaporate to dryness under reduced pressure in a water bath at 35 to 40 ° C, add 200 ml of acetone to the residue and stir at 10 to 15 ° C to analyze crystal. After filtration, the filter cake was dried under reduced pressure at 35-40°C to obtain Intermediate 1 with a yield of 97%, a purity of 99.89%, and a pH value of Intermediate 1 of 10.7. The molar ratio of disodium hydrogen phosphate to omeprazole is 1:1.
[0069] Step (2) preparation of intermediate 2
[0070] With embodiment 1.
[0071] Step (3): Preparation of Intermed...
Embodiment 3
[0079] Embodiment 3 The present embodiment is the embodiment of preparing esomeprazole sodium
[0080] Step (1) preparation of intermediate 1
[0081] With embodiment 1.
[0082] Step (2) preparation of intermediate 2
[0083] With embodiment 1.
[0084] Step (3): Preparation of Intermediate 3
[0085] With embodiment 1.
[0086] Step (4): Preparation of Intermediate 4
[0087] Add 3.5g of sodium hydroxide and 200ml of ethyl acetate to 324.2g of the intermediate obtained in step (3) and 200ml of purified water, stir for 2 hours, separate layers, acidify the alkaline aqueous layer, extract with ethyl acetate, dry, evaporate under reduced pressure The crude product of intermediate 4 esomeprazole was dried; the molar ratio of sodium hydroxide to intermediate 3 was 6:1; the yield of intermediate 4 was 97%, and the HPLC purity was 99.811%.
[0088] Step (5): Preparation of intermediate 5
[0089] With embodiment 1.
[0090] Step (6): Refining
[0091] Add ethanol and activ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com