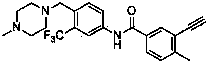Preparation method of drug for treating chronic granulocytic leukemia
A methyl and ethynyl technology, applied in the field of medicinal chemistry, can solve the problem of not being able to obtain the target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of 3-ethynyl-4-methyl-N-[4-(4-methylpiperazin-1-ylmethyl)-3-trifluoromethylphenyl]benzamide
[0047]
[0048] Step A: Preparation of 3-iodo-4-methyl-N-[4-(4-methylpiperazin-1-ylmethyl)-3-trifluoromethylphenyl]benzamide:
[0049] Add 3-iodo-4-methyl-benzoic acid (2.62g, 10mmol) and 10ml of thionyl chloride into a round bottom flask, reflux at 78°C for 4 hours, remove volatile substances by rotary evaporator to obtain 3-iodo-4 - Methyl-benzoyl chloride. In a round bottom flask was added 4-(4-methylpiperazin-1-ylmethyl)-3-trifluoromethylaniline (2.27 g, 8.3 mmol), 3-iodo-4-methyl-benzoyl chloride (10mmol), 15ml tetrahydrofuran, 10ml triethylamine, stirred at room temperature for 4 hours. with saturated NaHCO 3 Solution washing, adding ethyl acetate and water extraction, washing with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column ...
Embodiment 2
[0061]Example 2: Preparation of 3-ethynyl-4-methyl-N-[4-(4-methylpiperazin-1-ylmethyl)-3-trifluoromethylphenyl]benzamide
[0062]
[0063] Step A: Preparation of methyl 4-methyl-3-trimethylsilylethynylbenzoate
[0064] Methyl 4-methyl-3-iodobenzoate (15 g, 54.33 mmol), bistriphenylphosphine palladium dichloride (1.91 g, 2.72 mmol), cuprous iodide (1.03 g, 5.43 mmol) were added to In a 50ml round-bottomed two-neck flask, nitrogen was replaced three times. Under nitrogen protection, trimethylsilylacetylene (21.6mL, 162.99 mmol) and triethylamine (12.5 mL, 86.93 mmol) were added, heated to 60°C and stirred overnight. , TLC monitored the reaction and found that the raw material 4-methyl-3-iodobenzoic acid methyl ester was completely consumed, the reaction solution was evaporated under reduced pressure to remove the solvent, added ethyl acetate and water for extraction, combined the organic phases, washed with saturated brine, and anhydrous sodium sulfate dry. It was dried in ...
Embodiment 3
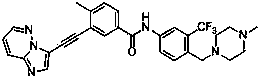
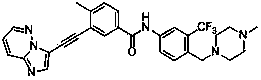
[0072] Example 3 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazine-1- Base) methyl] -3-trifluoromethylphenyl} benzamide
[0073]
[0074] Add 3-ethynyl-4-methyl-N-[4-(4-methylpiperazin-1-ylmethyl)-3-trifluoromethylphenyl]benzamide (126mg ,0.3mmol), 3-bromoimidazo[1,2-b]pyridazine (59mg,0.3mmol), Pd(PPh 3 ) 2 Cl 2 (11mg, 0.02mmol), PCy 3 (8mg, 0.04mmol), Cs 2 CO 3 (99mg, 0.3mmol), DBU 0.3ml, DMF 10ml, replace the air with argon for 5 minutes, seal, and stir at 80°C for 8 hours. Extract with ethyl acetate (15ml×4), combine the organic phases, add anhydrous Na 2 SO 4 dry. The organic solution was concentrated under reduced pressure, and the residue was purified by silica gel column to obtain the target compound with a yield of 58%.
[0075] 1 HNMR (CDCl 3 ,500MHz): 8.48(d,1H,Ar-H),8.31(s,1H,Ar-H),8.06(s,1H,-NH),8.06(s,1H,Ar-H), 7.98(d ,1H,Ar-H)7.93(d,1H,Ar-H),7.90(s,1H,Ar-H),7.83(q,1H,Ar-H),7.72(d,1H,Ar-H) ,7.38(d,1H,Ar-H),7.14(q,1H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com