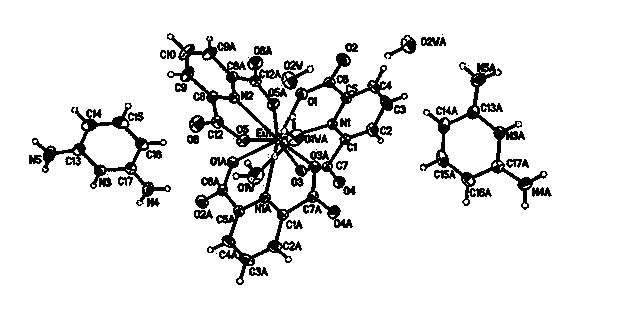
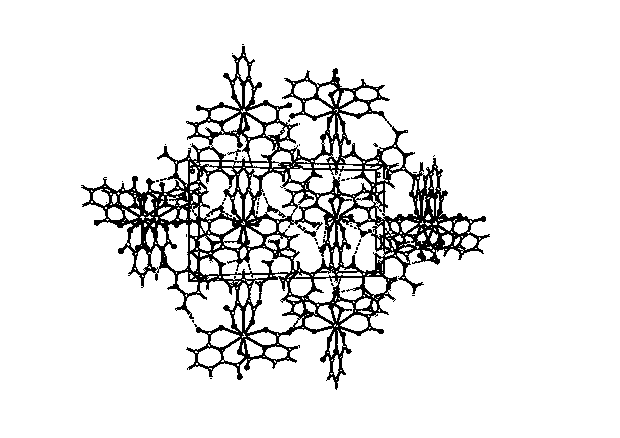
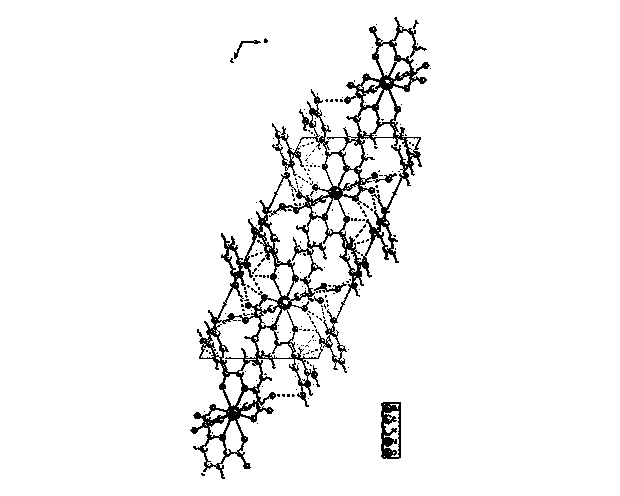
Hydrogen-bonded rare-earth metal europium complex fluorescent material and preparation method thereof
A rare earth metal, europium complex technology, applied in luminescent materials, chemical instruments and methods, compounds containing periodic table Group 3/13 elements, etc. Assembly and application in the field of fluorescent materials without giving structural information of rare earth metal complexes, etc., to achieve the effect of narrow emission peak, high fluorescence intensity and excellent fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 0.2676 g (0.6 mmol) of Eu(NO 3 ) 3 ·6H 2 O was dissolved in 50 mL of water to obtain solution A1; 0.2183 g (2 mmol) of 2,6-diaminopyridine and 0.3342 g (2 mmol) of pyridine-2,6-dicarboxylic acid were weighed and dissolved in 100 mL of ethanol solution, Stir and reflux at 60°C for 24 h, filter with suction, wash with ethanol three times, and dry in vacuum at 60°C for 4 h to obtain 0.4326 g (pydaH 2 )(pydc) proton transfer compound B1, yield 78.3%;
[0033] Weigh 0.3315 g (1.2 mmol) B1 and dissolve it in 30 mL water to obtain B1 solution; raise the temperature to 80°C, add A1 solution dropwise to B1 solution, stir and react for 5 h, filter, wash with ethanol three times, and dry at 60°C After 4 h, 0.2212 g of off-white solid powder was obtained, with a yield of 41.4%; the filtrate was slowly crystallized at room temperature to obtain pale yellow blocky crystal C1. C1 was washed three times with a small amount of water and ethanol, and filtered to obtain a comple...
Embodiment 2
[0035] Weigh 0.3664 g (1 mmol) of EuCl 3 ·6H 2 O was dissolved in 5 mL of ethanol to obtain solution A2; 0.2728 g (2.5 mmol) of 2,6-diaminopyridine and 0.4345 g (2.6 mmol) of pyridine-2,6-dicarboxylic acid were weighed and dissolved in 15 mL of water, 70 ℃, stirred and refluxed for 12 h, filtered with suction, washed with water three times, and dried in vacuum at 50 °C for 6 h to obtain 0.6451 g (pydaH 2 )(pydc) proton transfer compound B2, yield 91.2%;
[0036] Weigh 0.3315 g (1.2 mmol) of B2 and dissolve it in a mixed solvent of 10 mL of water and 50 mL of ethanol to obtain the B2 solution; raise the temperature to 50°C, add the A2 solution dropwise into the B2 solution, stir for 6 h, filter, and wash with ethanol After washing three times and drying at 50°C for 5 h, 0.2885 g of off-white solid powder was obtained, with a yield of 48.9%. The filtrate was slowly crystallized at room temperature to obtain light yellow blocky crystal C2. C2 was washed three times with a smal...
Embodiment 3
[0038] Weigh 0.3380 g (0.5 mmol) of Eu 2 (C 2 o 4 ) 3 ·6H 2 O was dissolved in 10 mL of water and 10 mL of ethanol mixed solvent to obtain solution A3; weigh 0.5238 g (4.8 mmol) of 2,6-diaminopyridine and 0.7688 g (4.6 mmol) of pyridine-2,6-dicarboxylic acid and dissolve in In 40 mL ethanol solution, stirred and refluxed at 50 °C for 20 h, filtered with suction, washed with ethanol three times, and dried in vacuum at 70 °C for 4 h to obtain 1.1737 g (pydaH 2 )(pydc) proton transfer compound B3, yield 90.8%;
[0039] Weigh 0.8288 g (3 mmol) B3 and dissolve it in the mixed solution of 10 mL water and 5 mL ethanol to obtain the B3 solution; warm up to 50 °C, add the A3 solution dropwise into the B3 solution, stir for 4 h, filter, and use After washing with absolute ethanol three times, drying at 60°C for 4 h, 0.5787 g of off-white solid powder was obtained, with a yield of 52.0%. The filtrate was slowly crystallized at room temperature to obtain C3 as pale yellow blocky crys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com