Preparation method of zirconium fluoride
A technology of zirconium fluoride and zirconium tetrafluoride, which is applied in the field of preparation of zirconium fluoride, can solve the problems of high raw material cost and complicated process, and achieve the effect of simple preparation method, wide sources and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The embodiment of the present invention provides a preparation method of zirconium fluoride, comprising:
[0026] mixing the zirconium-containing substance with the fluoride to obtain a mixture;
[0027] The mixture is reacted to obtain gaseous zirconium tetrafluoride and solid oxide;
[0028] Gas-solid separation to obtain gaseous zirconium tetrafluoride;
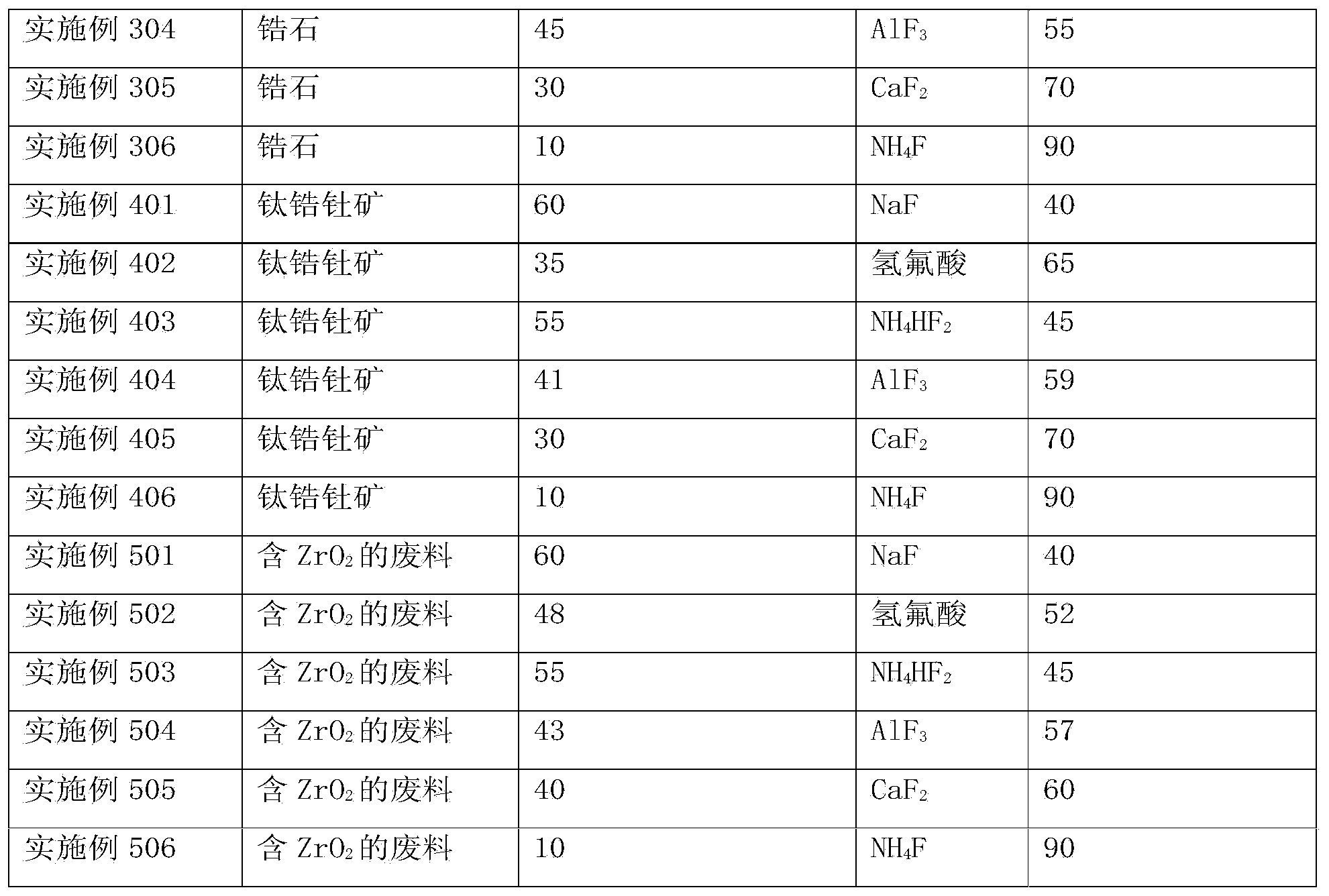
[0029] Wherein, the zirconium-containing substances include: zirconium-containing minerals or zirconium oxide-containing wastes.
[0030] Zirconium-containing substances (including zirconium-containing minerals or zirconium oxide-containing wastes) are used as raw materials, which have a wide range of sources and low cost; the zirconium in these raw materials is a fluorine-friendly substance, and after contacting with fluoride, it preferably reacts with fluorine to generate Zirconium tetrafluoride and other solid oxides can be separated by gas-solid separation to obtain zirconium tetrafluoride. Therefore, the pre...
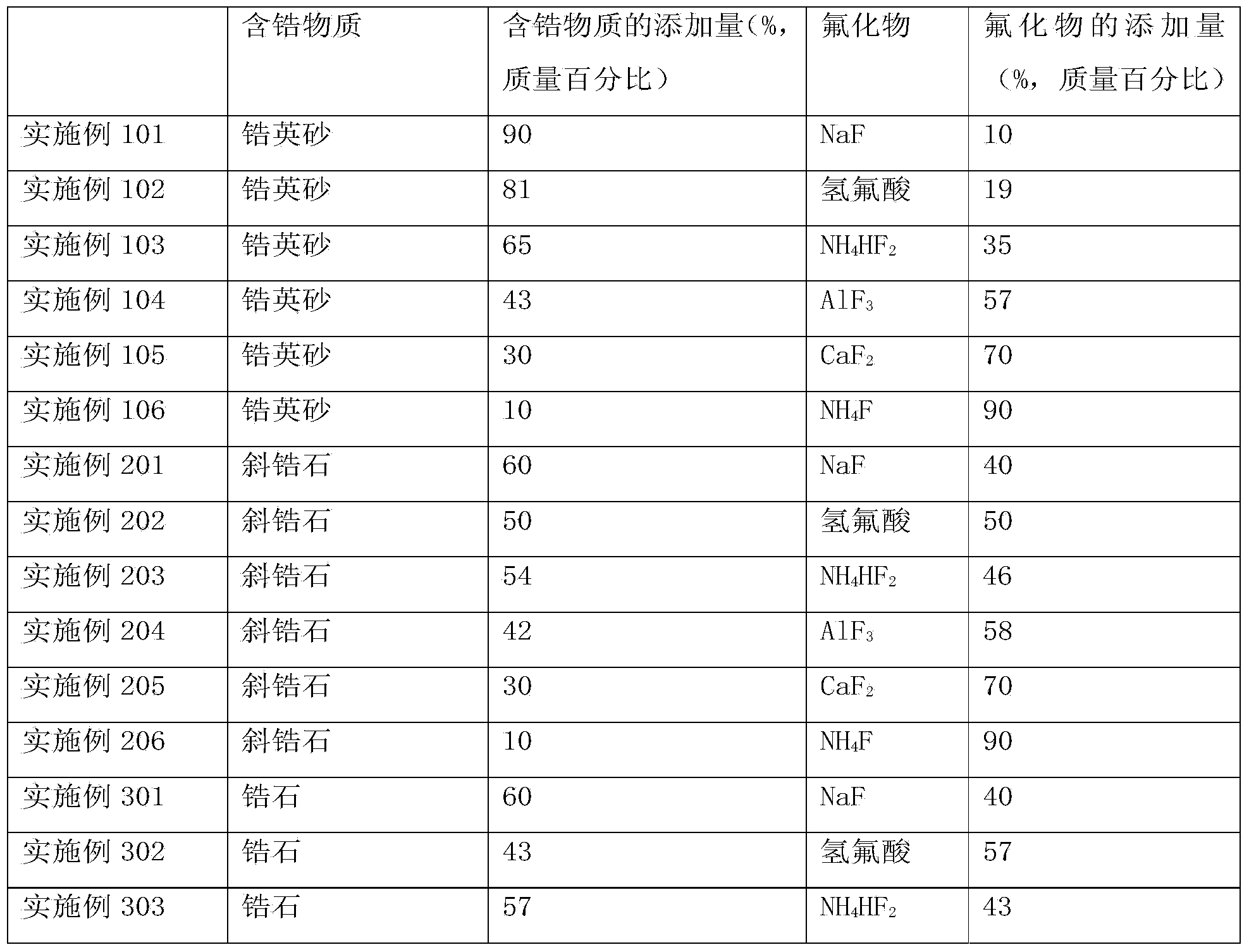
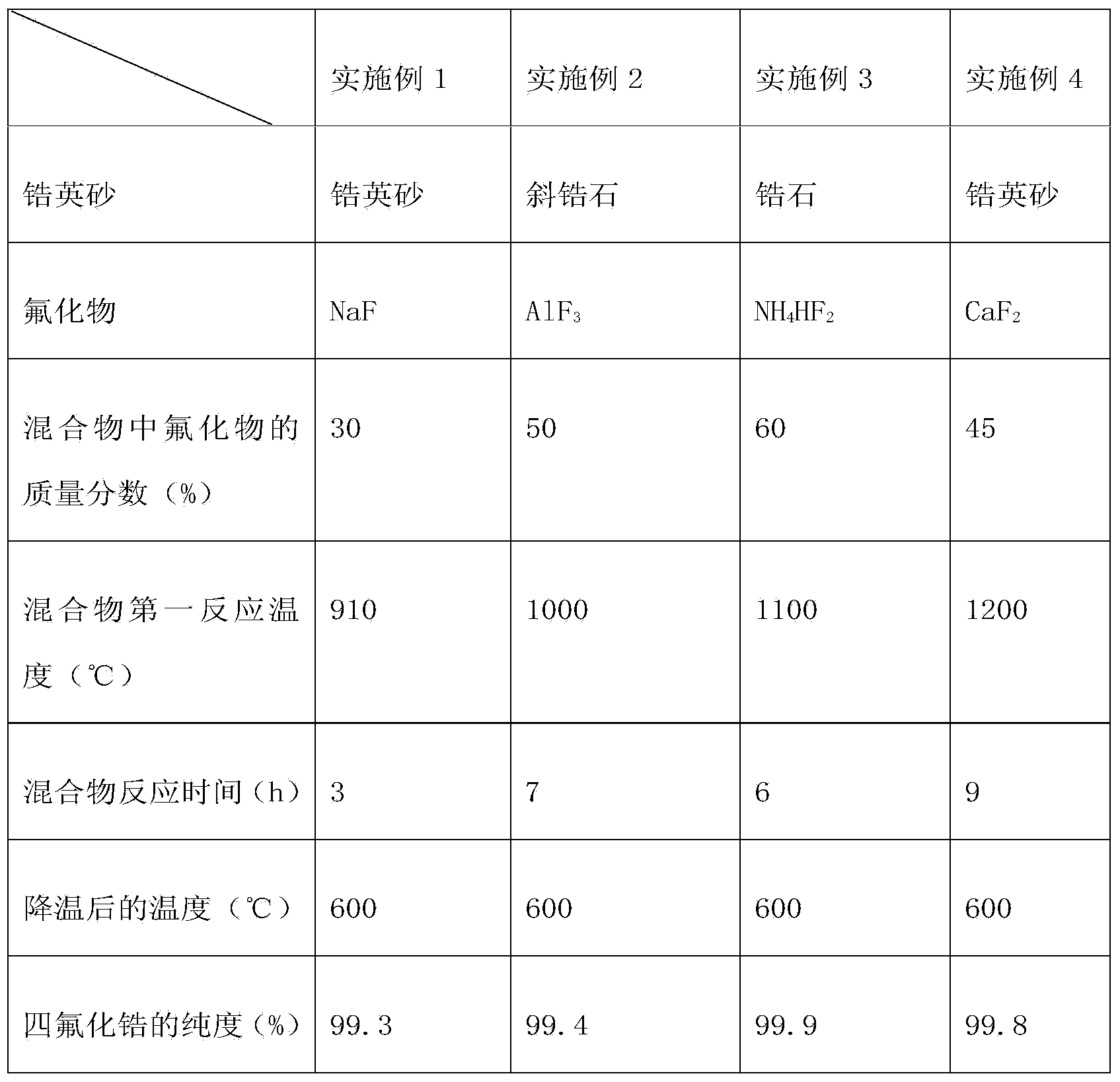
Embodiment 1
[0059] Example 1: Preparation of high-purity ZrF with zircon sand and NaF as raw materials 4
[0060] The zircon sand and NaF are mixed uniformly, the average particle size of silicon-containing metal oxide or silicon-containing mineral and fluoride is about 0.3 mm, and the mass percentage of NaF in the obtained mixture is 30%. The mixture was calcined at 910 °C for 3 hours to form Na 2 O, SiF 4 and ZrF 4 , SiF 4 , ZrF 4 At high temperature, it is gaseous and escapes from solid. The zirconium tetrafluoride and silicon tetrafluoride generated in the reactor are separated by gas-solid to obtain high-purity zirconium tetrafluoride and silicon tetrafluoride. Zirconium tetrafluoride. The reaction equation is as follows:
[0061] ZrO 2 +4NaF→ZrF 4 +2Na 2 O
[0062] SiO 2 +4NaF→2Na 2 O+SiF 4
Embodiment 2
[0063] Example 2: With baddeleyite and AlF 3 Preparation of high-purity ZrF as raw material 4
[0064] Combining baddeleyite with AlF 3 Mix well, baddeleyite zircon and AlF 3 The average particle size is about 0.9mm, and the AlF in the obtained mixture 3 The mass percentage is 50%. The mixture was calcined at 1000°C for 7 hours to form Al 2 O 3 and ZrF 4 , ZrF 4 It is gaseous at high temperature and escapes from solid. The zirconium tetrafluoride and silicon tetrafluoride generated in the reactor are separated by gas-solid to obtain high-purity zirconium tetrafluoride and silicon tetrafluoride. Zirconium tetrafluoride. The reaction equation is as follows:
[0065] 3ZrO 2 +4AlF 3 ·3H 2 O→2Al 2 O 3 +3ZrF 4 +12H 2 O
[0066] 3SiO 2 +4AlF 3 ·3H 2 O→2Al 2 O 3 +3SiF 4 +12H 2 O
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com